Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract
- PMID: 17360659
- PMCID: PMC1805557
- DOI: 10.1073/pnas.0607965104
Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract
Abstract
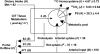
Methionine is an indispensable sulfur amino acid that functions as a key precursor for the synthesis of homocysteine and cysteine. Studies in adult humans suggest that splanchnic tissues convert dietary methionine to homocysteine and cysteine by means of transmethylation and transsulfuration, respectively. Studies in piglets show that significant metabolism of dietary indispensable amino acids occurs in the gastrointestinal tissues (GIT), yet the metabolic fate of methionine in GIT is unknown. We show here that 20% of the dietary methionine intake is metabolized by the GIT in piglets implanted with portal and arterial catheters and fed milk formula. Based on analyses from intraduodenal and intravenous infusions of [1-(13)C]methionine and [(2)H(3)]methionine, we found that the whole-body methionine transmethylation and remethylation rates were significantly higher during duodenal than intravenous tracer infusion. First-pass splanchnic metabolism accounted for 18% and 43% of the whole-body transmethylation and remethylation, respectively. Significant transmethylation and transsulfuration was demonstrated in the GIT, representing approximately 27% and approximately 23% of whole-body fluxes, respectively. The methionine used by the GIT was metabolized into homocysteine (31%), CO(2) (40%), or tissue protein (29%). Cystathionine beta-synthase mRNA and activity was present in multiple GITs, including intestinal epithelial cells, but was significantly lower than liver. We conclude that the GIT consumes 20% of the dietary methionine and is a significant site of net homocysteine production. Moreover, the GITs represent a significant site of whole-body transmethylation and transsulfuration, and these two pathways account for a majority of methionine used by the GITs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

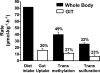

Similar articles
-
Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs.Am J Physiol Endocrinol Metab. 2009 Jun;296(6):E1239-50. doi: 10.1152/ajpendo.91021.2008. Epub 2009 Mar 17. Am J Physiol Endocrinol Metab. 2009. PMID: 19293331 Free PMC article.
-
Dietary methyl donors affect in vivo methionine partitioning between transmethylation and protein synthesis in the neonatal piglet.Amino Acids. 2016 Dec;48(12):2821-2830. doi: 10.1007/s00726-016-2317-x. Epub 2016 Aug 25. Amino Acids. 2016. PMID: 27562792
-
Moderate vitamin B-6 restriction does not alter postprandial methionine cycle rates of remethylation, transmethylation, and total transsulfuration but increases the fractional synthesis rate of cystathionine in healthy young men and women.J Nutr. 2011 May;141(5):835-42. doi: 10.3945/jn.110.134197. Epub 2011 Mar 23. J Nutr. 2011. PMID: 21430249 Free PMC article. Clinical Trial.
-
Intestinal metabolism of sulfur amino acids.Nutr Res Rev. 2009 Dec;22(2):175-87. doi: 10.1017/S0954422409990138. Nutr Res Rev. 2009. PMID: 19835653 Review.
-
Emerging aspects of gut sulfur amino acid metabolism.Curr Opin Clin Nutr Metab Care. 2007 Jan;10(1):63-8. doi: 10.1097/MCO.0b013e3280115d36. Curr Opin Clin Nutr Metab Care. 2007. PMID: 17143057 Review.
Cited by
-
Substitution of Dietary Sulfur Amino Acids by dl-2-Hydroxy-4-Methylthiobutyric Acid Reduces Fractional Glutathione Synthesis in Weaned Piglets.J Nutr. 2020 Apr 1;150(4):722-729. doi: 10.1093/jn/nxz272. J Nutr. 2020. PMID: 31773161 Free PMC article.
-
Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs.Am J Physiol Endocrinol Metab. 2009 Jun;296(6):E1239-50. doi: 10.1152/ajpendo.91021.2008. Epub 2009 Mar 17. Am J Physiol Endocrinol Metab. 2009. PMID: 19293331 Free PMC article.
-
Hydrogen Sulfide Sensing through Reactive Sulfur Species (RSS) and Nitroxyl (HNO) in Enterococcus faecalis.ACS Chem Biol. 2018 Jun 15;13(6):1610-1620. doi: 10.1021/acschembio.8b00230. Epub 2018 May 17. ACS Chem Biol. 2018. PMID: 29712426 Free PMC article.
-
Effect of L- or DL-methionine Supplementation on Nitrogen Retention, Serum Amino Acid Concentrations and Blood Metabolites Profile in Starter Pigs.Asian-Australas J Anim Sci. 2016 May;29(5):689-94. doi: 10.5713/ajas.15.0730. Epub 2016 Jan 18. Asian-Australas J Anim Sci. 2016. PMID: 26954214 Free PMC article.
-
Sulfur as a signaling nutrient through hydrogen sulfide.Annu Rev Nutr. 2014;34:171-205. doi: 10.1146/annurev-nutr-071813-105654. Annu Rev Nutr. 2014. PMID: 25033061 Free PMC article. Review.
References
-
- Brosnan JT, Brosnan ME. J Nutr. 2006;136:1636S–1640S. - PubMed
-
- Hogeveen M, Blom HJ, Van Amerongen M, Boogmans B, Van Beynum IM, Van De BM. J Pediatr. 2002;141:429–431. - PubMed
-
- Hobbs CA, Cleves MA, Melnyk S, Zhao W, James SJ. Am J Clin Nutr. 2005;81:147–153. - PubMed
-
- Selhub J. J Nutr. 2006;136(Suppl 6):1726S–1730S. - PubMed
-
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wilson PW, Wolf PA. N Engl J Med. 2002;346:476–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

