HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes
- PMID: 17360663
- PMCID: PMC1805607
- DOI: 10.1073/pnas.0611699104
HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes
Abstract
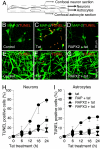
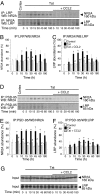
HIV infection of the central nervous system can result in neurologic dysfunction with devastating consequences in AIDS patients. NeuroAIDS is characterized by neuronal injury and loss, yet there is no evidence that HIV can infect neurons. Here we show that the HIV-encoded protein tat triggers formation of a macromolecular complex involving the low-density lipoprotein receptor-related protein (LRP), postsynaptic density protein-95 (PSD-95), N-methyl-d-aspartic acid (NMDA) receptors, and neuronal nitric oxide synthase (nNOS) at the neuronal plasma membrane, and that this complex leads to apoptosis in neurons negative as well as positive for NMDA receptors and also in astrocytes. Blockade of LRP-mediated tat uptake, NMDA receptor activation, or neuronal nitric oxide synthase significantly reduces ensuing neuronal apoptosis, suggesting that formation of this complex is an early step in tat toxicity. We also show that the inflammatory chemokine, CCL2, protects against tat toxicity and inhibits formation of the complex. These findings implicate the complex in HIV-induced neuronal apoptosis and suggest therapeutic targets for intervention in the pathogenesis of NeuroAIDS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis.J Neurochem. 2003 Jun;85(5):1299-311. doi: 10.1046/j.1471-4159.2003.01775.x. J Neurochem. 2003. PMID: 12753088
-
Astrocytes mediate HIV-1 Tat-induced neuronal damage via ligand-gated ion channel P2X7R.J Neurochem. 2015 Feb;132(4):464-76. doi: 10.1111/jnc.12953. J Neurochem. 2015. PMID: 25272052
-
Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands.Nat Med. 2000 Dec;6(12):1380-7. doi: 10.1038/82199. Nat Med. 2000. PMID: 11100124
-
HIV tat and neurotoxicity.Microbes Infect. 2006 Apr;8(5):1347-57. doi: 10.1016/j.micinf.2005.11.014. Epub 2006 Jan 26. Microbes Infect. 2006. PMID: 16697675 Review.
-
[HIV-1 neuropathogenesis: therapeutic strategies against neuronal loss induced by gp120/Tat glycoprotein in the central nervous system].Rev Neurol. 2011 Jan 16;52(2):101-11. Rev Neurol. 2011. PMID: 21271550 Review. Spanish.
Cited by
-
Human immunodeficiency virus-1 protein Tat induces excitotoxic loss of presynaptic terminals in hippocampal cultures.Mol Cell Neurosci. 2013 May;54:22-9. doi: 10.1016/j.mcn.2012.12.005. Epub 2012 Dec 23. Mol Cell Neurosci. 2013. PMID: 23267846 Free PMC article.
-
Integrated Analysis of the miRNA-mRNA Regulatory Network Involved in HIV-Associated Neurocognitive Disorder.Pathogens. 2022 Mar 27;11(4):407. doi: 10.3390/pathogens11040407. Pathogens. 2022. PMID: 35456082 Free PMC article.
-
HIV increases the release of dickkopf-1 protein from human astrocytes by a Cx43 hemichannel-dependent mechanism.J Neurochem. 2014 Mar;128(5):752-63. doi: 10.1111/jnc.12492. Epub 2013 Nov 13. J Neurochem. 2014. PMID: 24134157 Free PMC article.
-
Methods to study monocyte migration induced by HIV-infected cells.Methods Mol Biol. 2009;485:295-309. doi: 10.1007/978-1-59745-170-3_20. Methods Mol Biol. 2009. PMID: 19020833 Free PMC article.
-
Repeated cocaine treatment enhances HIV-1 Tat-induced cortical excitability via over-activation of L-type calcium channels.J Neuroimmune Pharmacol. 2014 Jun;9(3):354-68. doi: 10.1007/s11481-014-9524-6. Epub 2014 Feb 25. J Neuroimmune Pharmacol. 2014. PMID: 24567038 Free PMC article.
References
-
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. J Neuropathol Exp Neurol. 2005;64:529–536. - PubMed
-
- Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Nature. 1990;345:84–86. - PubMed
-
- Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. J Neurochem. 2001;78:457–467. - PubMed
-
- Wang P, Barks JD, Silverstein FS. Neuroscience. 1999;88:585–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS20752/NS/NINDS NIH HHS/United States
- AI-051519/AI/NIAID NIH HHS/United States
- P30 AI051519/AI/NIAID NIH HHS/United States
- NS11920/NS/NINDS NIH HHS/United States
- MH052974/MH/NIMH NIH HHS/United States
- 5 T32 GM007288/GM/NIGMS NIH HHS/United States
- NS45287/NS/NINDS NIH HHS/United States
- P20RR015592/RR/NCRR NIH HHS/United States
- R01 NS045287/NS/NINDS NIH HHS/United States
- P20 RR015592/RR/NCRR NIH HHS/United States
- R01 MH070297/MH/NIMH NIH HHS/United States
- MH075679/MH/NIMH NIH HHS/United States
- R01 NS020752/NS/NINDS NIH HHS/United States
- K01 MH076679/MH/NIMH NIH HHS/United States
- MH070297/MH/NIMH NIH HHS/United States
- P50 NS011920/NS/NINDS NIH HHS/United States
- R01 MH075679/MH/NIMH NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

