SseL, a Salmonella deubiquitinase required for macrophage killing and virulence
- PMID: 17360673
- PMCID: PMC1802004
- DOI: 10.1073/pnas.0610095104
SseL, a Salmonella deubiquitinase required for macrophage killing and virulence
Abstract
Expression of the Salmonella enterica serovar Typhimurium pathogenicity island 2 (SPI-2) type III secretion system is controlled by the two-component regulatory system SsrA-SsrB. We used a transcriptomic approach to help define the SsrA-SsrB regulon. We identified a gene encoding an uncharacterized effector (SseL) whose translocation into host cells depends on the SPI-2 secretion system. SseL has similarities to cysteine proteases with deubiquitinating activity. A GST-SseL fusion protein specifically hydrolyzed mono- and polyubiquitin substrates in vitro with a preference for K63-linked ubiquitin chains. Ubiquitin-modified proteins accumulated in macrophages infected with Salmonella sseL mutant strains but to a lesser extent when infected with bacteria expressing active protein, demonstrating that SseL functions as a deubiquitinase in vivo. Salmonella sseL mutant strains did not show a replication defect or induce altered levels of cytokine production upon infection of macrophages but were defective for a delayed cytotoxic effect and were attenuated for virulence in mice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
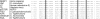
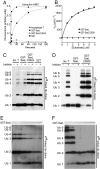
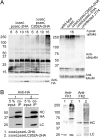
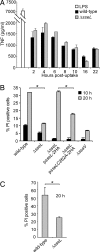
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

