Characterization of human immunodeficiency virus type 1 monomeric and trimeric gp120 glycoproteins stabilized in the CD4-bound state: antigenicity, biophysics, and immunogenicity
- PMID: 17360741
- PMCID: PMC1900256
- DOI: 10.1128/JVI.02500-06
Characterization of human immunodeficiency virus type 1 monomeric and trimeric gp120 glycoproteins stabilized in the CD4-bound state: antigenicity, biophysics, and immunogenicity
Abstract
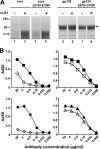
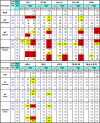
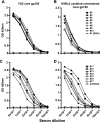
The human immunodeficiency virus type 1 exterior gp120 envelope glycoprotein is highly flexible, and this flexibility may contribute to the inability of monomeric gp120 immunogens to elicit broadly neutralizing antibodies. We previously showed that an S375W modification of a critical interfacial cavity central to the primary receptor binding site, the Phe43 cavity, stabilizes gp120 into the CD4-bound state. However, the immunological effects of this cavity-altering replacement were never tested. Subsequently, we screened other mutations that, along with the S375W alteration, might further stabilize the CD4-bound state. Here, we define a selected second cavity-altering replacement, T257S, and analyze the double mutations in several gp120 envelope glycoprotein contexts. The gp120 glycoproteins with the T257S-plus-S375W double mutation (T257S+S375W) have a superior antigenic profile compared to the originally identified single S375W replacement in terms of enhanced recognition by the broadly neutralizing CD4 binding-site antibody b12. Isothermal titration calorimetry measuring the entropy of the gp120 interaction with CD4 indicated that the double mutant was also stabilized into the CD4-bound state, with increasing relative fixation between core, full-length monomeric, and full-length trimeric versions of gp120. A significant increase in gp120 affinity for CD4 was also observed for the cavity-filling mutants relative to wild-type gp120. The most conformationally constrained T257S+S375W trimeric gp120 proteins were selected for immunogenicity analysis in rabbits and displayed a trend of improvement relative to their wild-type counterparts in terms of eliciting neutralizing antibodies. Together, the results suggest that conformational stabilization may improve the ability of gp120 to elicit neutralizing antibodies.
Figures





References
-
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
-
- Barnett, S. W., S. Rajasekar, H. Legg, B. Doe, D. H. Fuller, J. R. Haynes, C. M. Walker, and K. S. Steimer. 1997. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine 15:869-873. - PubMed
-
- Belshe, R. B., G. J. Gorse, M. J. Mulligan, T. G. Evans, M. C. Keefer, J. L. Excler, A. M. Duliege, J. Tartaglia, W. I. Cox, J. McNamara, K. L. Hwang, A. Bradney, D. Montefiori, K. J. Weinhold, et al. 1998. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS 12:2407-2415. - PubMed
-
- Berman, P. W., T. J. Gregory, L. Riddle, G. R. Nakamura, M. A. Champe, J. P. Porter, F. M. Wurm, R. D. Hershberg, E. K. Cobb, and J. W. Eichberg. 1990. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature 345:622-625. - PubMed
-
- Binley, J. M., R. Wyatt, E. Desjardins, P. D. Kwong, W. Hendrickson, J. P. Moore, and J. Sodroski. 1998. Analysis of the interaction of antibodies with a conserved enzymatically deglycosylated core of the HIV type 1 envelope glycoprotein 120. AIDS Res. Hum. Retrovir. 14:191-198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

