The E1circumflexE4 protein of human papillomavirus interacts with the serine-arginine-specific protein kinase SRPK1
- PMID: 17360743
- PMCID: PMC1900295
- DOI: 10.1128/JVI.02609-06
The E1circumflexE4 protein of human papillomavirus interacts with the serine-arginine-specific protein kinase SRPK1
Abstract
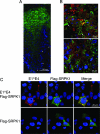
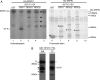
Human papillomavirus (HPV) infections of the squamous epithelium are associated with high-level expression of the E1circumflexE4 protein during the productive phase of infection. However, the precise mechanisms of how E1circumflexE4 contributes to the replication cycle of the virus are poorly understood. Here, we show that the serine-arginine (SR)-specific protein kinase SRPK1 is a novel binding partner of HPV type 1 (HPV1) E1circumflexE4. We map critical residues within an arginine-rich domain of HPV1 E1circumflexE4, and in a region known to facilitate E1circumflexE4 oligomerization, that are requisite for SRPK1 binding. In vitro kinase assays show that SRPK1 binding is associated with phosphorylation of an HPV1 E1circumflexE4 polypeptide and modulates autophosphorylation of the kinase. We show that SRPK1 is sequestered into E4 inclusion bodies in terminally differentiated cells within HPV1 warts and that colocalization between E1circumflexE4 and SRPK1 is not dependent on additional HPV1 factors. Moreover, we also identify SRPK1 binding of E1circumflexE4 proteins of HPV16 and HPV18. Our findings indicate that SRPK1 binding is a conserved function of E1circumflexE4 proteins of diverse virus types. SRPK1 influences important biochemical processes within the cell, including nuclear organization and RNA metabolism. While phosphorylation of HPV1 E4 by SRPK1 may directly influence HPV1 E4 function during the infectious cycle, the modulation and sequestration of SRPK1 by E1circumflexE4 may affect the ability of SRPK1 to phosphorylate its cellular targets, thereby facilitating the productive phase of the HPV replication cycle.
Figures





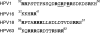
Similar articles
-
Human papillomavirus type 1 E1^E4 protein is a potent inhibitor of the serine-arginine (SR) protein kinase SRPK1 and inhibits phosphorylation of host SR proteins and of the viral transcription and replication regulator E2.J Virol. 2014 Nov;88(21):12599-611. doi: 10.1128/JVI.02029-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142587 Free PMC article.
-
The human papillomavirus type 11 E1E4 protein is phosphorylated in genital epithelium.Virology. 2000 Mar 15;268(2):430-9. doi: 10.1006/viro.1999.0173. Virology. 2000. PMID: 10704351
-
The splicing factor kinase, SR protein kinase 1 (SRPK1) is essential for late events in the human papillomavirus life cycle.PLoS Pathog. 2025 Apr 9;21(4):e1012697. doi: 10.1371/journal.ppat.1012697. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40203066 Free PMC article.
-
Serine arginine protein kinase 1 (SRPK1): a moonlighting protein with theranostic ability in cancer prevention.Mol Biol Rep. 2019 Feb;46(1):1487-1497. doi: 10.1007/s11033-018-4545-5. Epub 2018 Dec 8. Mol Biol Rep. 2019. PMID: 30535769 Review.
-
Serine-arginine protein kinases and their targets in viral infection and their inhibition.Cell Mol Life Sci. 2023 May 17;80(6):153. doi: 10.1007/s00018-023-04808-6. Cell Mol Life Sci. 2023. PMID: 37198350 Free PMC article. Review.
Cited by
-
Phosphorylation events during viral infections provide potential therapeutic targets.Rev Med Virol. 2012 May;22(3):166-81. doi: 10.1002/rmv.722. Epub 2011 Nov 24. Rev Med Virol. 2012. PMID: 22113983 Free PMC article.
-
Interplay Between CMGC Kinases Targeting SR Proteins and Viral Replication: Splicing and Beyond.Front Microbiol. 2021 Mar 29;12:658721. doi: 10.3389/fmicb.2021.658721. eCollection 2021. Front Microbiol. 2021. PMID: 33854493 Free PMC article. Review.
-
Analysis of CpG methylation sites and CGI among human papillomavirus DNA genomes.BMC Genomics. 2011 Nov 25;12:580. doi: 10.1186/1471-2164-12-580. BMC Genomics. 2011. PMID: 22118413 Free PMC article.
-
RNA Binding Proteins that Control Human Papillomavirus Gene Expression.Biomolecules. 2015 May 5;5(2):758-74. doi: 10.3390/biom5020758. Biomolecules. 2015. PMID: 25950509 Free PMC article. Review.
-
Molecular Mechanism of SR Protein Kinase 1 Inhibition by the Herpes Virus Protein ICP27.mBio. 2019 Oct 22;10(5):e02551-19. doi: 10.1128/mBio.02551-19. mBio. 2019. PMID: 31641093 Free PMC article.
References
-
- Ashmole, I., P. H. Gallimore, and S. Roberts. 1998. Identification of conserved hydrophobic C-terminal residues of the human papillomavirus type 1 E1E4 protein necessary for E4 oligomerisation in vivo. Virology 240:221-231. - PubMed
-
- Breitburd, F., O. Croissant, and G. Orth. 1987. Expression of human papillomavirus type-1 E4 gene products in warts, p. 115-122. In B. M. Steinberg, J. Brandsma, and L. B. Taichman (ed.), Papillomaviruses, vol. 5. Cold Spring Harbor Press, Cold Spring Harbor, NY.
-
- Brown, D. R., D. Kitchin, B. Qadadri, N. Neptune, T. Batteiger, and A. Ermel. 2006. The human papillomavirus type 11 E1-E4 protein is a transglutaminase 3 substrate and induces abnormalities of the cornified cell envelope. Virology 345:290-298. - PubMed
-
- Bryan, J. T., A. Han, K. H. Fife, and D. R. Brown. 2000. The human papillomavirus type 11 E1E4 protein is phosphorylated in genital epithelium. Virology 268:430-439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

