Lack of the murine homeobox gene Hesx1 leads to a posterior transformation of the anterior forebrain
- PMID: 17360769
- PMCID: PMC2233881
- DOI: 10.1242/dev.02829
Lack of the murine homeobox gene Hesx1 leads to a posterior transformation of the anterior forebrain
Abstract
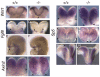
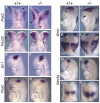
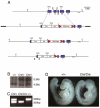
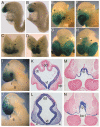
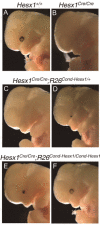
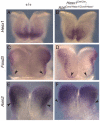
The homeobox gene Hesx1 is an essential repressor that is required within the anterior neural plate for normal forebrain development in mouse and humans. Combining genetic cell labelling and marker analyses, we demonstrate that the absence of Hesx1 leads to a posterior transformation of the anterior forebrain (AFB) during mouse development. Our data suggest that the mechanism underlying this transformation is the ectopic activation of Wnt/beta-catenin signalling within the Hesx1 expression domain in the AFB. When ectopically expressed in the developing mouse embryo, Hesx1 alone cannot alter the normal fate of posterior neural tissue. However, conditional expression of Hesx1 within the AFB can rescue the forebrain defects observed in the Hesx1 mutants. The results presented here provide new insights into the function of Hesx1 in forebrain formation.
Figures

References
-
- Brickman JM, Clements M, Tyrell R, McNay D, Woods K, Warner J, Stewart A, Beddington RS, Dattani MT. Molecular effects of novel mutations in Hesx1/HESX1 associated with human pituitary disorders. Development. 2001;128:5189–5199. - PubMed
-
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev. Biol. 1987;120:198–214. - PubMed
-
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

