Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury
- PMID: 17360828
- PMCID: PMC2117896
- DOI: 10.1152/jn.01168.2006
Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury
Abstract
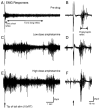
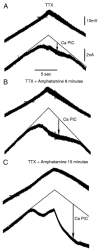
The recovery of persistent inward currents (PICs) and motoneuron excitability after chronic spinal cord transection is mediated, in part, by the development of supersensitivity to residual serotonin (5HT) below the lesion. The purpose of this paper is to investigate if, like 5HT, endogenous sources of norepinephrine (NE) facilitate motoneuron PICs after chronic spinal transection. Cutaneous-evoked reflex responses in tail muscles of awake chronic spinal rats were measured after increasing presynaptic release of NE by administration of amphetamine. An increase in long-lasting reflexes, known to be mediated by the calcium component of the PIC (CaPIC), was observed even at low doses (0.1-0.2 mg/kg) of amphetamine. These findings were repeated in a reduced S2 in vitro preparation, demonstrating that the increased long-lasting reflexes by amphetamine were neural. Under intracellular voltage clamp, amphetamine application led to a large facilitation of the motoneuron CaPIC. This indicates that the increases in long-lasting reflexes induced by amphetamine in the awake animal were, in part, due to actions directly on the motoneuron. Reflex responses in acutely spinal animals were facilitated by amphetamine similar to chronic animals but only at doses that were ten times greater than that required in chronic animals (0.2 mg/kg chronic vs. 2.0 mg/kg acute), pointing to a development of supersensitivity to endogenous NE in chronic animals. In summary, the increases in long-lasting reflexes and associated motoneuron CaPICs by amphetamine are likely due to an increased release of endogenous NE, which motoneurons become supersensitive to in the chronic stages of spinal cord injury.
Figures

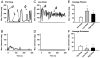
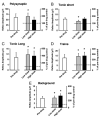
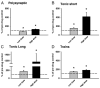
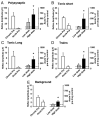
 ) and high (▪, right of graphs) doses of amphetamine for the various reflex responses. Dashed line indicates 100% of predrug values. A: polysynaptic reflex (10–40 ms after stimulus): predrug reflex is 60.9 ± 16.7 μV and shows a significant increase of 194.1 ± 48.6% at low doses of amphetamine and an increase of 145.7 ± 41.5% at high doses. B: tonic short reflex (30–600 ms after stim): predrug reflex is 12.8 ± 1.7 μV and shows significant increase at low (129.7 ± 15.9%) but not high (162.5 ± 20.8%) doses of amphetamine. C: tonic long reflex (600–3,600 ms after stim): predrug reflex is 9.4 ± 3.8 μV, and increases by 429.2 ± 145.6% at low and by 392.3 ± 140.2% at high doses of amphetamine. D: trains reflex (1,100–4,100 s after 500 ms, 100-Hz train stim): predrug reflex is 12.8 ± 3.6 μV and increases significantly by 262.8 ± 54.2% at low dose and 270.0 ± 70.1% at high doses of amphetamine. E: background activity: predrug levels of activity measure 4.4 ± 1.0 μV and increase by 270.4 ± 57.1% at low dose and 330.2 ± 58.6% at high dose amphetamine. * = P < 0.05.
) and high (▪, right of graphs) doses of amphetamine for the various reflex responses. Dashed line indicates 100% of predrug values. A: polysynaptic reflex (10–40 ms after stimulus): predrug reflex is 60.9 ± 16.7 μV and shows a significant increase of 194.1 ± 48.6% at low doses of amphetamine and an increase of 145.7 ± 41.5% at high doses. B: tonic short reflex (30–600 ms after stim): predrug reflex is 12.8 ± 1.7 μV and shows significant increase at low (129.7 ± 15.9%) but not high (162.5 ± 20.8%) doses of amphetamine. C: tonic long reflex (600–3,600 ms after stim): predrug reflex is 9.4 ± 3.8 μV, and increases by 429.2 ± 145.6% at low and by 392.3 ± 140.2% at high doses of amphetamine. D: trains reflex (1,100–4,100 s after 500 ms, 100-Hz train stim): predrug reflex is 12.8 ± 3.6 μV and increases significantly by 262.8 ± 54.2% at low dose and 270.0 ± 70.1% at high doses of amphetamine. E: background activity: predrug levels of activity measure 4.4 ± 1.0 μV and increase by 270.4 ± 57.1% at low dose and 330.2 ± 58.6% at high dose amphetamine. * = P < 0.05.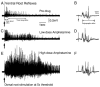



Similar articles
-
Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity.J Neurophysiol. 2004 Nov;92(5):2694-703. doi: 10.1152/jn.00164.2004. J Neurophysiol. 2004. PMID: 15486423
-
Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury.J Neurophysiol. 2007 Feb;97(2):1236-46. doi: 10.1152/jn.00995.2006. Epub 2006 Nov 1. J Neurophysiol. 2007. PMID: 17079337 Free PMC article.
-
Spastic long-lasting reflexes of the chronic spinal rat studied in vitro.J Neurophysiol. 2004 May;91(5):2236-46. doi: 10.1152/jn.01010.2003. J Neurophysiol. 2004. PMID: 15069101
-
Persistent inward currents in spinal motoneurons: important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis.Clin Neurophysiol. 2010 Oct;121(10):1669-79. doi: 10.1016/j.clinph.2009.12.041. Epub 2010 May 11. Clin Neurophysiol. 2010. PMID: 20462789 Free PMC article. Review.
-
Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns.Neuroscientist. 2008 Jun;14(3):264-75. doi: 10.1177/1073858408314986. Epub 2008 Apr 1. Neuroscientist. 2008. PMID: 18381974 Free PMC article. Review.
Cited by
-
Noradrenergic innervation of the rat spinal cord caudal to a complete spinal cord transection: effects of olfactory ensheathing glia.Exp Neurol. 2010 Mar;222(1):59-69. doi: 10.1016/j.expneurol.2009.12.008. Epub 2009 Dec 16. Exp Neurol. 2010. PMID: 20025875 Free PMC article.
-
Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection?J Physiol. 2008 Jan 15;586(2):529-44. doi: 10.1113/jphysiol.2007.141499. Epub 2007 Nov 15. J Physiol. 2008. PMID: 18006586 Free PMC article.
-
Effect of prolonged riluzole exposure on cultured motoneurons in a mouse model of ALS.J Neurophysiol. 2012 Jan;107(1):484-92. doi: 10.1152/jn.00714.2011. Epub 2011 Oct 19. J Neurophysiol. 2012. PMID: 22013234 Free PMC article.
-
Increasing motor neuron excitability to treat weakness in sepsis.Ann Neurol. 2017 Dec;82(6):961-971. doi: 10.1002/ana.25105. Epub 2017 Dec 7. Ann Neurol. 2017. PMID: 29171917 Free PMC article.
-
Vibration-induced extra torque during electrically-evoked contractions of the human calf muscles.J Neuroeng Rehabil. 2010 Jun 10;7:26. doi: 10.1186/1743-0003-7-26. J Neuroeng Rehabil. 2010. PMID: 20537167 Free PMC article.
References
-
- Aboul-Enein HY. Amphetamine: a pharmacological profile. Am J Pharmacol Sci Support Public Health. 1971;143:161–164. - PubMed
-
- Alaburda A, Perrier JF, Hounsgaard J. Mechanisms causing plateau potentials in spinal motoneurons. Adv Exp Med Biol. 2002;508:219–226. - PubMed
-
- Anderson RJ, Brigham P, Cady WJ, Kellar KJ. Role of alpha-2 adrenergic affinity in the action of a non-sedative antispasticity agent. Acta Neurol Scand. 1982;66:248–258. - PubMed
-
- Austin JH, Nygren LG, Fuxe K. A system for measuring the noradrenaline receptor contribution to the flexor reflex. Med Biol. 1976;54:352–363. - PubMed
-
- Barbeau H, Bedard P. Denervation supersensitivity to 5-hydroxytryptophan in rats following spinal transection and 5,7-dihydroxytryptamine injection. Neuropharmacology. 1981;20:611–616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

