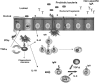
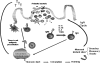
Proposed model: mechanisms of immunomodulation induced by probiotic bacteria
- PMID: 17360855
- PMCID: PMC1865623
- DOI: 10.1128/CVI.00406-06
Proposed model: mechanisms of immunomodulation induced by probiotic bacteria
Figures


References
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. - PubMed
-
- Axelsson, L. T., T. C. Chung, W. G. Dobrogosz, and S. E. Lindgren. 1989. Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb. Ecol. Health Dis. 2:131-136.
-
- Berg, R. D. 1998. Probiotic, probiotics or ‘conbiotics’? Trends Microbiol. 6:89-92. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

