Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer's disease
- PMID: 17360894
- PMCID: PMC6672562
- DOI: 10.1523/JNEUROSCI.3758-06.2007
Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer's disease
Abstract
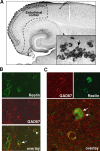
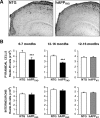
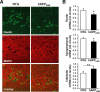
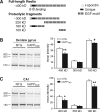
Reelin regulates nervous system development and modulates synaptic plasticity in the adult brain. Several findings suggest that alterations in Reelin signaling may contribute to neuronal dysfunction associated with Alzheimer's disease (AD). Cell surface receptors for Reelin, including integrins and very-low-density lipoprotein receptor/apolipoprotein E2 receptor, may be targets of amyloid-beta (Abeta) peptides presumed to play key roles in the pathogenesis of AD. Reelin also regulates the extent of tau phosphorylation. Finally, increased amounts of Reelin fragments have been found in CSF from AD patients, suggesting altered processing of Reelin. We therefore hypothesized that Reelin levels might be altered in the brains of human amyloid precursor protein (hAPP) transgenic mice, particularly in brain regions vulnerable to AD such as hippocampus and entorhinal cortex. Compared with nontransgenic controls, hAPP mice had significantly fewer Reelin-expressing pyramidal cells in the entorhinal cortex, the major population of glutamatergic neurons expressing Reelin in the brain. Western blot analysis of the hippocampus, which receives projections from the entorhinal cortex, revealed significant reductions in Reelin levels. In contrast, the number of Reelin-expressing GABAergic interneurons was not altered in either the entorhinal cortex or the hippocampus. Thus, neuronal expression of hAPP/Abeta is sufficient to reduce Reelin expression in a specific population of entorhinal cortical pyramidal neurons in vivo. Underscoring the relevance of these findings, we found qualitatively similar reductions of Reelin-expressing pyramidal neurons in the entorhinal cortex of AD brains. We conclude that alterations in Reelin processing or signaling may be involved in AD-related neuronal dysfunction.
Figures
References
-
- Beall MJ, Lewis DA. Heterogeneity of layer II neurons in human entorhinal cortex. J Comp Neurol. 1992;321:241–266. - PubMed
-
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. - PubMed
-
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. - PubMed
-
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
