Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation
- PMID: 17360895
- PMCID: PMC6672591
- DOI: 10.1523/JNEUROSCI.3849-06.2007
Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation
Abstract
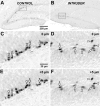
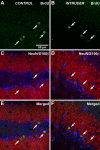
Factors modulating neurogenesis may contribute to the pathophysiology of affective disorders such as major depression. Environmental stressors in animal models have been proposed to alter neurogenesis, suggesting a mechanism for this contribution. The effect of an acute psychosocial stressor on either proliferation or survival (immediate, short term, and long term) was examined along with subsequent neuronal differentiation in the hippocampus of adult male Sprague Dawley rats. Subjects were exposed to a widely used social dominance paradigm that elicits behavioral and physiological responses to an acute psychosocial stressor. This social dominance paradigm may mimic human relational stress more realistically than laboratory stressors and provides a socially relevant model. We found that exposure to an acute psychosocial stressor at the time of cell generation resulted in a decreased number of newly generated cells in the hippocampus. By using sequential thymidine analog administration to provide temporal discrimination of DNA replication, we showed that short-term survival but not initial proliferation or immediate survival was altered in response to stress. Furthermore, we determined that stress experienced subsequent to proliferation also diminished long-term survival of cells. Thus, an acute episode of a social stress produces long-lasting effects on the incorporation of new hippocampal neurons by reducing their survival.
Figures



Similar articles
-
Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus.Exp Neurol. 2006 Oct;201(2):308-15. doi: 10.1016/j.expneurol.2006.04.010. Epub 2006 Jun 5. Exp Neurol. 2006. PMID: 16750196
-
Sex-dependent regulation of hippocampal neurogenesis under basal and chronic stress conditions in rats.Hippocampus. 2013 Jun;23(6):476-87. doi: 10.1002/hipo.22107. Epub 2013 Mar 18. Hippocampus. 2013. PMID: 23504963
-
Chronic administration of quetiapine stimulates dorsal hippocampal proliferation and immature neurons of male rats, but does not reverse psychosocial stress-induced hyponeophagic behavior.Psychiatry Res. 2019 Feb;272:411-418. doi: 10.1016/j.psychres.2018.12.137. Epub 2018 Dec 27. Psychiatry Res. 2019. PMID: 30611957
-
Psychosocial stress, glucocorticoids, and structural alterations in the tree shrew hippocampus.Physiol Behav. 2001 Jun;73(3):285-91. doi: 10.1016/s0031-9384(01)00497-8. Physiol Behav. 2001. PMID: 11438353 Review.
-
The Impact of Ethologically Relevant Stressors on Adult Mammalian Neurogenesis.Brain Sci. 2019 Jul 4;9(7):158. doi: 10.3390/brainsci9070158. Brain Sci. 2019. PMID: 31277460 Free PMC article. Review.
Cited by
-
BrdU birth dating can produce errors in cell fate specification in chick brain development.J Histochem Cytochem. 2012 Nov;60(11):801-10. doi: 10.1369/0022155412458588. Epub 2012 Aug 2. J Histochem Cytochem. 2012. PMID: 22859704 Free PMC article.
-
Estradiol-17beta-induced human neural progenitor cell proliferation is mediated by an estrogen receptor beta-phosphorylated extracellularly regulated kinase pathway.Endocrinology. 2008 Jan;149(1):208-18. doi: 10.1210/en.2007-1155. Epub 2007 Oct 25. Endocrinology. 2008. PMID: 17962344 Free PMC article.
-
Perinatal stress and human hippocampal volume: Findings from typically developing young adults.Sci Rep. 2018 Mar 16;8(1):4696. doi: 10.1038/s41598-018-23046-6. Sci Rep. 2018. PMID: 29549289 Free PMC article.
-
Hippocampal metabolic differences implicate distinctions between physical and psychological stress in four rat models of depression.Transl Psychiatry. 2018 Jan 10;8(1):4. doi: 10.1038/s41398-017-0018-1. Transl Psychiatry. 2018. PMID: 29317595 Free PMC article.
-
Prenatal stress induces long-term effects in cell turnover in the hippocampus-hypothalamus-pituitary axis in adult male rats.PLoS One. 2011;6(11):e27549. doi: 10.1371/journal.pone.0027549. Epub 2011 Nov 9. PLoS One. 2011. PMID: 22096592 Free PMC article.
References
-
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. - PubMed
-
- Benninghoff J, Schmitt A, Mossner R, Lesch KP. When cells become depressed: focus on neural stem cells in novel treatment strategies against depression. J Neural Transm. 2002;9:947–962. - PubMed
-
- Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001;73:261–271. - PubMed
-
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. - PubMed
-
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
