Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation
- PMID: 17360897
- PMCID: PMC6672573
- DOI: 10.1523/JNEUROSCI.3316-06.2007
Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation
Abstract
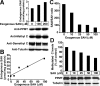
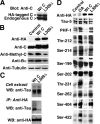
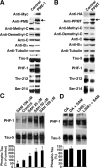
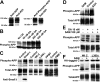
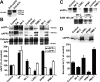
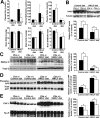
Alzheimer's disease (AD) neuropathology is characterized by the accumulation of phosphorylated tau and amyloid-beta peptides derived from the amyloid precursor protein (APP). Elevated blood levels of homocysteine are a significant risk factor for many age-related diseases, including AD. Impaired homocysteine metabolism favors the formation of S-adenosylhomocysteine, leading to inhibition of methyltransferase-dependent reactions. Here, we show that incubation of neuroblastoma cells with S-adenosylhomocysteine results in reduced methylation of protein phosphatase 2A (PP2A), a major brain Ser/Thr phosphatase, most likely by inhibiting PP2A methyltransferase (PPMT). PP2A methylation levels are also decreased after ectopic expression of PP2A methylesterase in Neuro-2a (N2a) cells. Reduced PP2A methylation promotes the downregulation of B alpha-containing holoenzymes, thereby affecting PP2A substrate specificity. It is associated with the accumulation of both phosphorylated tau and APP isoforms and increased secretion of beta-secretase-cleaved APP fragments and amyloid-beta peptides. Conversely, incubation of N2a cells with S-adenosylmethionine and expression of PPMT enhance PP2A methylation. This leads to the accumulation of dephosphorylated tau and APP species and increased secretion of neuroprotective alpha-secretase-cleaved APP fragments. Remarkably, hyperhomocysteinemia induced in wild-type and cystathionine-beta-synthase +/- mice by feeding a high-methionine, low-folate diet is associated with increased brain S-adenosylhomocysteine levels, PPMT downregulation, reduced PP2A methylation levels, and tau and APP phosphorylation. We reported previously that downregulation of neuronal PPMT and PP2A methylation occur in affected brain regions from AD patients. The link between homocysteine, PPMT, PP2A methylation, and key CNS proteins involved in AD pathogenesis provides new mechanistic insights into this disorder.
Figures
Similar articles
-
Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis.J Neuropathol Exp Neurol. 2004 Oct;63(10):1080-91. doi: 10.1093/jnen/63.10.1080. J Neuropathol Exp Neurol. 2004. PMID: 15535135
-
Disturbances in PP2A methylation and one-carbon metabolism compromise Fyn distribution, neuritogenesis, and APP regulation.J Biol Chem. 2021 Jan-Jun;296:100237. doi: 10.1074/jbc.RA120.016069. Epub 2021 Jan 7. J Biol Chem. 2021. PMID: 33380425 Free PMC article.
-
Folate deficiency induces in vitro and mouse brain region-specific downregulation of leucine carboxyl methyltransferase-1 and protein phosphatase 2A B(alpha) subunit expression that correlate with enhanced tau phosphorylation.J Neurosci. 2008 Nov 5;28(45):11477-87. doi: 10.1523/JNEUROSCI.2816-08.2008. J Neurosci. 2008. PMID: 18987184 Free PMC article.
-
Protein phosphatase 2A dysfunction in Alzheimer's disease.Front Mol Neurosci. 2014 Mar 11;7:16. doi: 10.3389/fnmol.2014.00016. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24653673 Free PMC article. Review.
-
Effects of carboxyl-terminal methylation on holoenzyme function of the PP2A subfamily.Biochem Soc Trans. 2020 Oct 30;48(5):2015-2027. doi: 10.1042/BST20200177. Biochem Soc Trans. 2020. PMID: 33125487 Free PMC article. Review.
Cited by
-
Epigenetic differences in cortical neurons from a pair of monozygotic twins discordant for Alzheimer's disease.PLoS One. 2009 Aug 12;4(8):e6617. doi: 10.1371/journal.pone.0006617. PLoS One. 2009. PMID: 19672297 Free PMC article.
-
Untangling Tau and Iron: Exploring the Interaction Between Iron and Tau in Neurodegeneration.Front Mol Neurosci. 2018 Aug 17;11:276. doi: 10.3389/fnmol.2018.00276. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30174587 Free PMC article. Review.
-
One-Carbon Metabolism: Pulling the Strings behind Aging and Neurodegeneration.Cells. 2022 Jan 9;11(2):214. doi: 10.3390/cells11020214. Cells. 2022. PMID: 35053330 Free PMC article. Review.
-
A chemical proteomic atlas of brain serine hydrolases identifies cell type-specific pathways regulating neuroinflammation.Elife. 2016 Jan 18;5:e12345. doi: 10.7554/eLife.12345. Elife. 2016. PMID: 26779719 Free PMC article.
-
Folic acid supplementation improves cognitive function by reducing the levels of peripheral inflammatory cytokines in elderly Chinese subjects with MCI.Sci Rep. 2016 Nov 23;6:37486. doi: 10.1038/srep37486. Sci Rep. 2016. PMID: 27876835 Free PMC article.
References
-
- Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem. 2001;276:40353–40361. - PubMed
-
- Arendt T, Holzer M, Fruth R, Bruckner MK, Gartner U. Phosphorylation of tau, Abeta-formation, and apoptosis after in vivo inhibition of PP-1 and PP-2A. Neurobiol Aging. 1998;19:3–13. - PubMed
-
- Baharians Z, Schonthal AH. Autoregulation of protein phosphatase type 2A expression. J Biol Chem. 1998;273:19019–19024. - PubMed
-
- Bottiglieri T. The effect of storage on rat tissues and human plasma amino acid levels determined by HPLC. Biomed Chromatogr. 1987;2:195–196. - PubMed
-
- Bottiglieri T. Isocratic high performance liquid chromatographic analysis of S-adenosylmethionine and S-adenosylhomo-cysteine in animal tissues: the effect of exposure to nitrous oxide. Biomed Chromatogr. 1990;4:239–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
