Status epilepticus-induced somatostatinergic hilar interneuron degeneration is regulated by striatal enriched protein tyrosine phosphatase
- PMID: 17360923
- PMCID: PMC2701360
- DOI: 10.1523/JNEUROSCI.4913-06.2007
Status epilepticus-induced somatostatinergic hilar interneuron degeneration is regulated by striatal enriched protein tyrosine phosphatase
Abstract
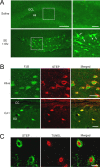
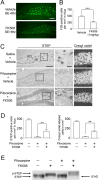
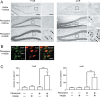
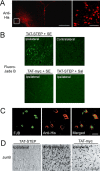
Excitotoxic cell death is one of the precipitating events in the development of temporal lobe epilepsy. Of particular prominence is the loss of GABAergic hilar neurons. Although the molecular mechanisms responsible for the selective vulnerability of these cells are not well understood, activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) pathway has been implicated in neuroprotective responses to excitotoxicity in other neuronal populations. Here, we report that high levels of the striatal-enriched protein tyrosine phosphatase (STEP), a key regulator of ERK/MAPK signaling, are found in vulnerable somatostatin-immunoreactive hilar interneurons. Under both control conditions and after pilocarpine-induced status epilepticus (SE), ERK/MAPK activation was repressed in STEP-immunoreactive hilar neurons. This contrasts with robust SE-induced ERK/MAPK activation in the granule cell layer of the dentate gyrus, a cell region that does not express STEP. During pilocarpine-induced SE, in vivo disruption of STEP activity allowed activation of the MAPK pathway, leading to immediate-early gene expression and significant rescue from cell death. Thus, STEP increases the sensitivity of neurons to SE-induced excitotoxicity by specifically blocking a latent neuroprotective response initiated by the MAPK pathway. These findings identify a key set of signaling events that render somatostatinergic hilar interneurons vulnerable to SE-induced cell death.
Figures



Similar articles
-
Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy.Epilepsia. 2011 Nov;52(11):2057-64. doi: 10.1111/j.1528-1167.2011.03253.x. Epub 2011 Aug 29. Epilepsia. 2011. PMID: 21883182 Free PMC article.
-
Degeneration and proliferation of astrocytes in the mouse dentate gyrus after pilocarpine-induced status epilepticus.Exp Neurol. 2006 Oct;201(2):416-27. doi: 10.1016/j.expneurol.2006.04.031. Epub 2006 Jun 21. Exp Neurol. 2006. PMID: 16793040 Free PMC article.
-
Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy.J Comp Neurol. 2007 Feb 10;500(5):876-93. doi: 10.1002/cne.21207. J Comp Neurol. 2007. PMID: 17177260 Free PMC article.
-
Hilar somatostatin interneuron loss reduces dentate gyrus inhibition in a mouse model of temporal lobe epilepsy.Epilepsia. 2016 Jun;57(6):977-83. doi: 10.1111/epi.13376. Epub 2016 Mar 31. Epilepsia. 2016. PMID: 27030321 Free PMC article.
-
Reduced excitatory drive onto interneurons in the dentate gyrus after status epilepticus.J Neurosci. 2001 Mar 15;21(6):2048-57. doi: 10.1523/JNEUROSCI.21-06-02048.2001. J Neurosci. 2001. PMID: 11245688 Free PMC article.
Cited by
-
An Excitatory and Epileptogenic Effect of Dentate Gyrus Mossy Cells in a Mouse Model of Epilepsy.Cell Rep. 2019 Nov 26;29(9):2875-2889.e6. doi: 10.1016/j.celrep.2019.10.100. Cell Rep. 2019. PMID: 31775052 Free PMC article.
-
CREB is a key regulator of striatal vulnerability in chemical and genetic models of Huntington's disease.Neurobiol Dis. 2009 Nov;36(2):259-68. doi: 10.1016/j.nbd.2009.07.014. Epub 2009 Jul 24. Neurobiol Dis. 2009. PMID: 19632326 Free PMC article.
-
The enigmatic mossy cell of the dentate gyrus.Nat Rev Neurosci. 2016 Sep;17(9):562-75. doi: 10.1038/nrn.2016.87. Epub 2016 Jul 28. Nat Rev Neurosci. 2016. PMID: 27466143 Free PMC article. Review.
-
In vitro stretch injury induces time- and severity-dependent alterations of STEP phosphorylation and proteolysis in neurons.J Neurotrauma. 2012 Jul 1;29(10):1982-98. doi: 10.1089/neu.2011.2253. Epub 2012 Jun 25. J Neurotrauma. 2012. PMID: 22435660 Free PMC article.
-
Profiling status epilepticus-induced changes in hippocampal RNA expression using high-throughput RNA sequencing.Sci Rep. 2014 Nov 6;4:6930. doi: 10.1038/srep06930. Sci Rep. 2014. PMID: 25373493 Free PMC article.
References
-
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. - PubMed
-
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. - PubMed
-
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
