The in vivo function of a noncanonical TRAF2-binding domain in the C-terminus of CD40 in driving B-cell growth and differentiation
- PMID: 17360936
- PMCID: PMC1896113
- DOI: 10.1182/blood-2006-07-038414
The in vivo function of a noncanonical TRAF2-binding domain in the C-terminus of CD40 in driving B-cell growth and differentiation
Abstract
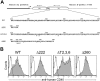
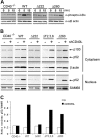
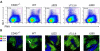
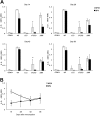
The recruitment of tumor necrosis factor receptor-associated factors (TRAFs) 1, 2, 3, 5, and 6 to the CD40 cytoplasmic tail upon CD40 trimerization results in downstream signaling events that ultimately lead to CD40-dependent, thymus-dependent (TD) humoral immune responses. Previously, we have shown signaling through the C-terminal tail of CD40 in the absence of canonical TRAF-binding sites is capable of signaling through an alternative TRAF2-binding site. Here, we demonstrate that B cells from mice harboring CD40 with only the C-terminal tail can activate both canonical and noncanonical NFkappaB signaling pathways. Moreover, while lacking germinal center formation, several hallmarks of humoral immune responses including clonal B-cell activation/expansion, antibody isotype switching, and affinity maturation remain normal. This study demonstrates a new functional domain in CD40 that controls critical aspects of B-cell immunity in an in vivo setting.
Figures
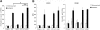
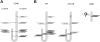
Similar articles
-
CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). Binding site specificity and activation of downstream pathways by distinct TRAFs.J Biol Chem. 1999 May 14;274(20):14246-54. doi: 10.1074/jbc.274.20.14246. J Biol Chem. 1999. PMID: 10318845
-
Roles of the TRAF2/3 binding site in differential B cell signaling by CD40 and its viral oncogenic mimic, LMP1.J Immunol. 2009 Sep 1;183(5):2966-73. doi: 10.4049/jimmunol.0900442. Epub 2009 Aug 10. J Immunol. 2009. PMID: 19667091 Free PMC article.
-
Role of tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2) in distinct and overlapping CD40 and TNF receptor 2/CD120b-mediated B lymphocyte activation.J Biol Chem. 2004 Dec 17;279(51):53222-31. doi: 10.1074/jbc.M410539200. Epub 2004 Oct 12. J Biol Chem. 2004. PMID: 15485859
-
Role of TRAFs in Signaling Pathways Controlling T Follicular Helper Cell Differentiation and T Cell-Dependent Antibody Responses.Front Immunol. 2018 Oct 22;9:2412. doi: 10.3389/fimmu.2018.02412. eCollection 2018. Front Immunol. 2018. PMID: 30405612 Free PMC article. Review.
-
CD40-CD154 interactions in B-cell signaling.Curr Top Microbiol Immunol. 2000;245(2):73-99. doi: 10.1007/978-3-642-59641-4_4. Curr Top Microbiol Immunol. 2000. PMID: 10533319 Review. No abstract available.
Cited by
-
TRAF2 and TRAF3 independently mediate Ig class switching driven by CD40.Int Immunol. 2009 Apr;21(4):477-88. doi: 10.1093/intimm/dxp013. Epub 2009 Feb 19. Int Immunol. 2009. PMID: 19228877 Free PMC article.
-
Suppression by Δ(9)-tetrahydrocannabinol of the primary immunoglobulin M response by human peripheral blood B cells is associated with impaired STAT3 activation.Toxicology. 2013 Aug 9;310:84-91. doi: 10.1016/j.tox.2013.05.009. Epub 2013 May 29. Toxicology. 2013. PMID: 23727458 Free PMC article.
-
cIAP1-dependent TRAF2 degradation regulates the differentiation of monocytes into macrophages and their response to CD40 ligand.Blood. 2009 Jan 1;113(1):175-85. doi: 10.1182/blood-2008-02-137919. Epub 2008 Sep 30. Blood. 2009. PMID: 18827186 Free PMC article.
-
The role of CD40/CD40 ligand interactions in bone marrow granulopoiesis.ScientificWorldJournal. 2011;11:2011-9. doi: 10.1100/2011/671453. Epub 2011 Oct 26. ScientificWorldJournal. 2011. PMID: 22125452 Free PMC article. Review.
-
A large cohort from an immunology reference center and an algorithm for the follow-up of chronic neutropenia.J Clin Immunol. 2024 Nov 5;45(1):38. doi: 10.1007/s10875-024-01816-4. J Clin Immunol. 2024. PMID: 39499404
References
-
- Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. - PubMed
-
- Cheng G, Cleary AM, Ye ZS, Hong DI, Lederman S, Baltimore D. Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science. 1995;267:1494–1498. - PubMed
-
- Sato T, Irie S, Reed JC. A novel member of the TRAF family of putative signal transducing proteins binds to the cytosolic domain of CD40. FEBS Lett. 1995;358:113–118. - PubMed
-
- Hu HM, O'Rourke K, Boguski MS, Dixit VM. A novel RING finger protein interacts with the cytoplasmic domain of CD40. J Biol Chem. 1994;269:30069–30072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

