Dynactin is required for coordinated bidirectional motility, but not for dynein membrane attachment
- PMID: 17360970
- PMCID: PMC1877108
- DOI: 10.1091/mbc.e06-08-0695
Dynactin is required for coordinated bidirectional motility, but not for dynein membrane attachment
Abstract
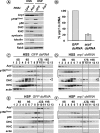
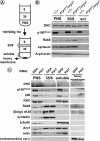
Transport of cellular and neuronal vesicles, organelles, and other particles along microtubules requires the molecular motor protein dynein (Mallik and Gross, 2004). Critical to dynein function is dynactin, a multiprotein complex commonly thought to be required for dynein attachment to membrane compartments (Karki and Holzbaur, 1999). Recent work also has found that mutations in dynactin can cause the human motor neuron disease amyotrophic lateral sclerosis (Puls et al., 2003). Thus, it is essential to understand the in vivo function of dynactin. To test directly and rigorously the hypothesis that dynactin is required to attach dynein to membranes, we used both a Drosophila mutant and RNA interference to generate organisms and cells lacking the critical dynactin subunit, actin-related protein 1. Contrary to expectation, we found that apparently normal amounts of dynein associate with membrane compartments in the absence of a fully assembled dynactin complex. In addition, anterograde and retrograde organelle movement in dynactin deficient axons was completely disrupted, resulting in substantial changes in vesicle kinematic properties. Although effects on retrograde transport are predicted by the proposed function of dynactin as a regulator of dynein processivity, the additional effects we observed on anterograde transport also suggest potential roles for dynactin in mediating kinesin-driven transport and in coordinating the activity of opposing motors (King and Schroer, 2000).
Figures


References
-
- Almenar-Queralt A., Goldstein L. S. Linkers, packages and pathways: new concepts in axonal transport. Curr. Opin. Neurobiol. 2001;11:550–557. - PubMed
-
- Bowman A. B., Kamal A., Ritchings B. W., Philp A. V., McGrail M., Gindhart J. G., Goldstein L. S. Kinesin-dependent axonal transport is mediated by the Sunday driver (SYD) protein. Cell. 2000;103:583–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

