Optical spectroscopic studies of light-harvesting by pigment-reconstituted peridinin-chlorophyll-proteins at cryogenic temperatures
- PMID: 17361463
- PMCID: PMC1769343
- DOI: 10.1007/s11120-006-9090-8
Optical spectroscopic studies of light-harvesting by pigment-reconstituted peridinin-chlorophyll-proteins at cryogenic temperatures
Abstract
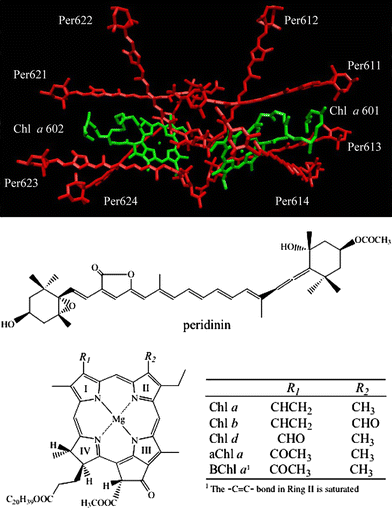
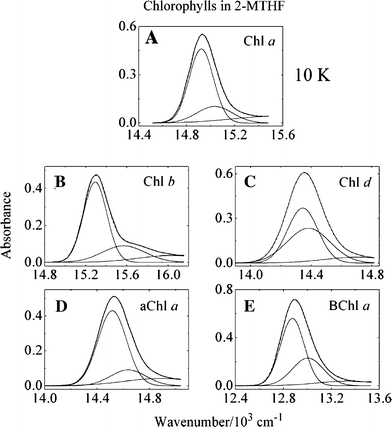
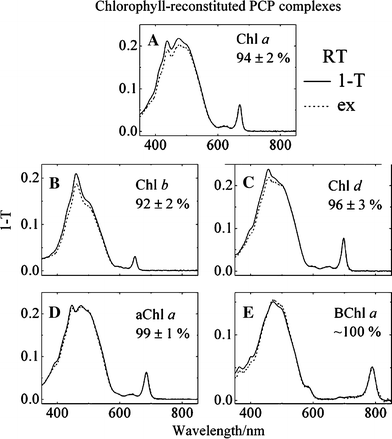
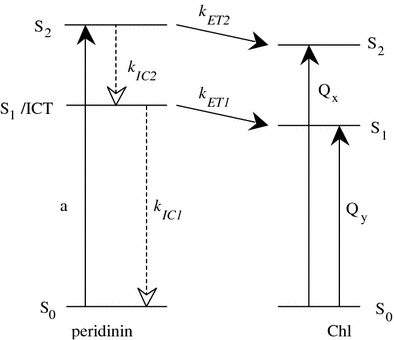
Low temperature, steady-state, optical spectroscopic methods were used to study the spectral features of peridinin-chlorophyll-protein (PCP) complexes in which recombinant apoprotein has been refolded in the presence of peridinin and either chlorophyll a (Chl a), chlorophyll b (Chl b), chlorophyll d (Chl d), 3-acetyl-chlorophyll a (3-acetyl-Chl a) or bacteriochlorophyll a (BChl a). Absorption spectra taken at 10 K provide better resolution of the spectroscopic bands than seen at room temperature and reveal specific pigment-protein interactions responsible for the positions of the Qy bands of the chlorophylls. The study reveals that the functional groups attached to Ring I of the two protein-bound chlorophylls modulate the Qy and Soret transition energies. Fluorescence excitation spectra were used to compute energy transfer efficiencies of the various complexes at room temperature and these were correlated with previously reported ultrafast, time-resolved optical spectroscopic dynamics data. The results illustrate the robust nature and value of the PCP complex, which maintains a high efficiency of antenna function even in the presence of non-native chlorophyll species, as an effective tool for elucidating the molecular details of photosynthetic light-harvesting.
Figures





References
-
- None
- Akahane J, Fiedor L, Rondonuwu FS, Watanabe Y, Koyama Y (2004) Reconstitution of the LH1 antenna complex from Rhodospirillum rubrum G9 and wild type by the use of five carotenoids having different conjugation lengths. Plant Cell Physiol 45:S100
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/0009-2614(96)00863-9', 'is_inner': False, 'url': 'https://doi.org/10.1016/0009-2614(96)00863-9'}]}
- Akimoto S, Takaichi S, Ogata T, Nishimura Y, Yamazaki I, Mimuro M (1996) Excitation energy transfer in carotenoid–chlorophyll protein complexes probed by femtosecond fluorescence decays. Chem Phys Lett 260:147–152
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/jp9916135', 'is_inner': False, 'url': 'https://doi.org/10.1021/jp9916135'}]}
- Bautista JA, Connors RE, Raju BB, Hiller RG, Sharples FP, Gosztola D, Wasielewski MR, Frank HA (1999) Excited state properties of peridinin: observation of a solvent dependence of the lowest excited singlet state lifetime and spectral behavior unique among carotenoids. J Phys Chem B 103:8751–8758
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1021/jp983943f', 'is_inner': False, 'url': 'https://doi.org/10.1021/jp983943f'}]}
- Bautista JA, Hiller RG, Sharples FP, Gosztola D, Wasielewski M, Frank HA (1999) Singlet and triplet energy transfer in the peridinin-chlorophyll a-protein from Amphidinium carterae. J Phys Chem A 103:2267–2273
-
- None
- Cammarata KV, Plumley FG, Schmidt GF (1990) Reconstitution of light-harvesting complexes: a single apoprotein binds Chl a, Chl b, and xanthophylls. In: Baltscheffsky M (eds) Current research in photosynthesis, vol. 2. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 341–344
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

