Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations
- PMID: 17362472
- PMCID: PMC1920547
- DOI: 10.1111/j.1742-1241.2007.01338.x
Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations
Abstract
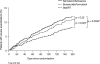
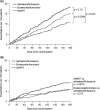
This randomised, double-blind, 6-month study compared budesonide/formoterol for maintenance and relief with salmeterol/fluticasone and a fixed maintenance dose of budesonide/formoterol, both with terbutaline for relief. Following a 2-week run-in, 3335 symptomatic adults and adolescents (mean FEV1 73% predicted, mean inhaled corticosteroid dose 745 microg/day) received budesonide/formoterol 160/4.5 microg one inhalation bid plus additional inhalations as needed, salmeterol/fluticasone 25/125 microg two inhalations bid plus as-needed terbutaline or budesonide/formoterol 320/9 microg one inhalation bid plus as-needed terbutaline. Budesonide/formoterol for maintenance and relief prolonged the time to first severe exacerbation requiring hospitalisation, emergency room treatment or oral steroids (primary variable) vs. fixed-dose salmeterol/fluticasone and budesonide/formoterol (p=0.0034 and p=0.023 respectively; log-rank test). Exacerbation rates were 19, 16 and 12 events/100 patients/6 months for salmeterol/fluticasone, fixed-dose budesonide/formoterol and budesonide/formoterol for maintenance and relief, respectively, [rate reduction vs. fixed-dose salmeterol/fluticasone (0.61; 95% CI 0.49-0.76, p<0.001) and vs. fixed-dose budesonide/formoterol (0.72; 95% CI 0.57-0.90, p=0.0048)]. Budesonide/formoterol maintenance and relief patients used less inhaled corticosteroid vs. salmeterol/fluticasone and fixed-dose budesonide/formoterol patients. All treatments provided similar marked improvements in lung function, asthma control days and asthma-related quality of life. Budesonide/formoterol for maintenance and relief reduces asthma exacerbations and maintains similar daily asthma control at a lower overall drug load compared with fixed-dose salmeterol/fluticasone and budesonide/formoterol.
Figures


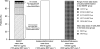
References
-
- Aalbers R, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed-dose salmeterol/fluticasone in moderate to severe asthma. Curr Med Res Opin. 2004;20:225–40. - PubMed
-
- Lalloo UG, et al. Budesonide and formoterol in a single inhaler improves asthma control compared with increasing the dose of corticosteroid in adults with mild-to-moderate asthma. Chest. 2003;123:1480–7. - PubMed
-
- Shapiro G, et al. Combined salmeterol 50 μg and fluticasone propionate 250 μg in the diskus device for the treatment of asthma. Am J Respir Crit Care Med. 2000;161:527–34. - PubMed
-
- Rabe KF, et al. Worldwide severity and control of asthma in children and adults: the global asthma insights and reality surveys. J Allergy Clin Immunol. 2004;114:40–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

