Influence of direct and indirect allorecognition pathways on CD4+CD25+ regulatory T-cell function in transplantation
- PMID: 17362475
- PMCID: PMC4329766
- DOI: 10.1111/j.1432-2277.2007.00470.x
Influence of direct and indirect allorecognition pathways on CD4+CD25+ regulatory T-cell function in transplantation
Abstract
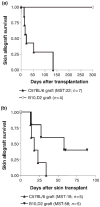
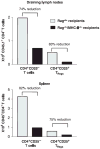
While both direct and indirect allorecognition are involved in allograft rejection, evidence to date suggests that tolerance is primarily dependent on indirect pathway-triggered CD4+CD25+ T cell-mediated immunoregulation. However, the precise influence of these two pathways on CD4+CD25+ T-cell function has not been addressed. In the current study, we have utilized an adoptive transfer model to assess selectively how the absence of either direct or indirect allorecognition affects CD4+CD25+ T-cell function. The effects of the loss of the direct pathway were assessed by transplanting skin grafts from minor histocompatibility mismatched B10.D2 (H-2d) donors onto Balb/c (H-2d) recipients, or by placing bone marrow chimeric DBA/2 (H-2d/H-2b) allografts onto C57BL/6 (H-2b) hosts. The requirement for indirect allorecognition was tested by grafting DBA/2 skin allografts onto either C57BL/6- or MHC-II-deficient C57BL/6 recipients. We report here that although CD4+CD25+ regulatory T cells can suppress both directly and indirectly generated alloresponses, immunoregulation is favored when indirect presentation is the sole mechanism of allorecognition. Hence, in the absence of indirect presentation, net CD4+CD25+ T cell-dependent immunoregulation is weak, and high ratios of CD4+CD25+ to CD4+CD25 T cells are required to ensure graft survival.
Figures


References
-
- Auchincloss H, Jr, Sultan H. Antigen processing and presentation in transplantation. Curr Opin Immunol. 1996;8:681. - PubMed
-
- Lombardi G, Sidhu S, Daly M, Batchelor JR, Makgoba W, Lechler RI. Are primary alloresponses truly primary? Int Immunol. 1990;2:9. - PubMed
-
- Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352. - PubMed
-
- Braun MY, Grandjean I, Feunou P, et al. Acute rejection in the absence of cognate recognition of allograft by T cells. J Immunol. 2001;166:4879. - PubMed
-
- Wise MP, Bemelman F, Cobbold SP, Waldmann H. Linked suppression of skin graft rejection can operate through indirect recognition. J Immunol. 1998;161:5813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

