The identification and characterization of a novel protein, c19orf10, in the synovium
- PMID: 17362502
- PMCID: PMC1906808
- DOI: 10.1186/ar2145
The identification and characterization of a novel protein, c19orf10, in the synovium
Abstract
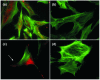
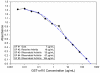
Joint inflammation and destruction have been linked to the deregulation of the highly synthetic fibroblast-like synoviocytes (FLSs), and much of our current understanding of the mechanisms that underlie synovitis has been collected from studies of FLSs. During a proteomic analysis of FLS cells, we identified a novel protein, c19orf10 (chromosome 19 open reading frame 10), that was produced in significant amounts by these cells. The present study provides a partial characterization of c19orf10 in FLSs, synovial fluid, and the synovium. Murine monoclonal and chicken polyclonal antibodies were produced against recombinant human c19orf10 protein and used to examine the distribution of c19orf10 in cultured FLSs and in synovial tissue sections from patients with rheumatoid arthritis or osteoarthritis. The intracellular staining pattern of c19orf10 is consistent with localization in the endoplasmic reticulum/Golgi distribution. Sections of rheumatoid arthritis and osteoarthritis synovia expressed similar patterns of c19orf10 distribution with perivascular and synovial lining staining. Double-staining in situ analysis suggests that fibroblast-like synovial cells produced c19orf10, whereas macrophages, B cells, or T cells produced little or none of this protein. There is evidence of secretion into the vascular space and the extracellular matrix surrounding the synovial lining. A competitive enzyme-linked immunosorbent assay confirmed the presence of microgram levels of c19orf10 in the synovial fluids of patients with one of various arthropathies. Collectively, these results suggest that c19orf10 is an FLS-derived protein that is secreted into the synovial fluid. However, the significance of this protein in synovial biology remains to be determined. The absence of known structural motifs or domains and its relatively late evolutionary appearance raise interesting questions about its function.
Figures





Similar articles
-
Comparison of cathepsins K and S expression within the rheumatoid and osteoarthritic synovium.Arthritis Rheum. 2002 Mar;46(3):663-74. doi: 10.1002/art.10114. Arthritis Rheum. 2002. PMID: 11920402
-
Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes.Arthritis Res Ther. 2006;8(6):R171. doi: 10.1186/ar2080. Arthritis Res Ther. 2006. PMID: 17105646 Free PMC article.
-
Production of cartilage oligomeric matrix protein (COMP) by cultured human dermal and synovial fibroblasts.Osteoarthritis Cartilage. 1998 Nov;6(6):435-40. doi: 10.1053/joca.1998.0147. Osteoarthritis Cartilage. 1998. PMID: 10343777
-
The synovium as a privileged site in rheumatoid arthritis: cadherin-11 as a dominant player in synovial pathology.Best Pract Res Clin Rheumatol. 2011 Dec;25(6):767-77. doi: 10.1016/j.berh.2011.11.012. Best Pract Res Clin Rheumatol. 2011. PMID: 22265259 Review.
-
Where to Stand with Stromal Cells and Chronic Synovitis in Rheumatoid Arthritis?Cells. 2019 Oct 15;8(10):1257. doi: 10.3390/cells8101257. Cells. 2019. PMID: 31618926 Free PMC article. Review.
Cited by
-
Crystal structure and receptor-interacting residues of MYDGF - a protein mediating ischemic tissue repair.Nat Commun. 2019 Nov 26;10(1):5379. doi: 10.1038/s41467-019-13343-7. Nat Commun. 2019. PMID: 31772377 Free PMC article.
-
C19orf10 promotes malignant behaviors of human bladder carcinoma cells via regulating the PI3K/AKT and Wnt/β-catenin pathways.J Cancer. 2021 May 19;12(14):4341-4354. doi: 10.7150/jca.56993. eCollection 2021. J Cancer. 2021. PMID: 34093834 Free PMC article.
-
Hypoxia-induced myeloid derived growth factor promotes hepatocellular carcinoma progression through remodeling tumor microenvironment.Theranostics. 2021 Jan 1;11(1):209-221. doi: 10.7150/thno.49327. eCollection 2021. Theranostics. 2021. PMID: 33391471 Free PMC article.
-
Myeloid-derived growth factor in diseases: structure, function and mechanisms.Mol Med. 2024 Jul 19;30(1):103. doi: 10.1186/s10020-024-00874-z. Mol Med. 2024. PMID: 39030488 Free PMC article. Review.
-
Phenotypic and proteomic characteristics of human dental pulp derived mesenchymal stem cells from a natal, an exfoliated deciduous, and an impacted third molar tooth.Stem Cells Int. 2014;2014:457059. doi: 10.1155/2014/457059. Epub 2014 Oct 14. Stem Cells Int. 2014. PMID: 25379041 Free PMC article.
References
-
- Tulin EE, Onoda N, Nakata Y, Maeda M, Hasegawa M, Nomura H, Kitamura T. SF20/IL-25, a novel bone marrow stroma-derived growth factor that binds to mouse thymic shared antigen-1 and supports lymphoid cell proliferation. J Immunol. 2001;167:6338–6347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

