Receptor mechanism and antiemetic activity of structurally-diverse cannabinoids against radiation-induced emesis in the least shrew
- PMID: 17362921
- PMCID: PMC1949344
- DOI: 10.1016/j.ejphar.2007.01.093
Receptor mechanism and antiemetic activity of structurally-diverse cannabinoids against radiation-induced emesis in the least shrew
Abstract
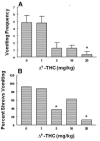
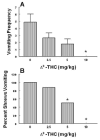
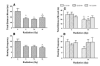
Xenobiotic cannabinoid CB1/CB2-receptor agonists appear to possess broad-spectrum antiemetic activity since they prevent vomiting produced by a variety of emetic stimuli including the chemotherapeutic agent cisplatin, serotonin 5-HT3-receptor agonists, dopamine D2/D3-receptor agonists and morphine, via the stimulation of CB1-receptors. The purpose of this study was to evaluate whether structurally-diverse cannabinoids [Delta9-THC, (delta-9-tetrahydrocannabinol); (Delta8-THC, delta-8-tetrahydrocannabinol); WIN55,212-2, (R (+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)), methyl] pyrolol [1,2,3-de]-1,4 benzoxazinyl]-(1-naphthalenyl) methenone mesylate); and CP55,940, ((-)-3-[2-hydroxy-4-(1,1-dimethylheptyl]-4-[3-hydroxypropyl] cyclohexane-1-ol)), can prevent radiation-induced emesis. Exposure to total body radiation (0, 5, 7.5 and 10 Gy) caused robust emesis in the least shrew (Cryptotis parva) in a dose-dependent manner (ED50=5.99 (5.77-6.23) Gy) and all animals vomited at the highest tested dose of radiation. In addition, the radiation exposure reduced locomotor behaviors to a significant but mild degree in a non-dose-dependent fashion up to one hour post-treatment. Radiation-induced emesis (10 Gy) was blocked in a dose-dependent manner by the CB1/CB2-receptor agonists with the following ID50 potency order: CP55,940 (0.11 (0.09-0.12) mg/kg)>WIN55,212,2 (3.65 (3.15-4.23) mg/kg)=Delta8-THC (4.36 (3.05-6.22) mg/kg)>Delta9-THC (6.76 (5.22-8.75) mg/kg). Although the greater antiemetic potency and efficacy of Delta8-THC relative to its isomer Delta9-THC is unusual as the latter cannabinoid possesses higher affinity and potency for cannabinoid receptors in functional assays, the current data support the results of a clinical study in children suggestive of complete protection from emesis by Delta8-THC. This effect has not been clinically observed for Delta9-THC in cancer patients receiving chemo- or radiation-therapy. Cannabinoids prevented the induced emesis via the stimulation of cannabinoid CB1-receptors because the CB1 (SR141716A)--and not the CB2 (SR144528)--receptor antagonist reversed both the observed reduction in emesis frequency and shrew emesis protection afforded by either Delta9-THC or CP55,940 against radiation-induced emesis. These findings further suggest that the least shrew can be utilized as a versatile and inexpensive small animal model to rapidly screen the efficacy of investigational antiemetics for the prevention of radiation-induced emesis.
Figures






Similar articles
-
Antiemetic and motor-depressive actions of CP55,940: cannabinoid CB1 receptor characterization, distribution, and G-protein activation.Eur J Pharmacol. 2003 Jan 10;459(1):83-95. doi: 10.1016/s0014-2999(02)02815-7. Eur J Pharmacol. 2003. PMID: 12505537
-
Delta-9-tetrahydrocannabinol differentially suppresses emesis versus enhanced locomotor activity produced by chemically diverse dopamine D2/D3 receptor agonists in the least shrew (Cryptotis parva).Pharmacol Biochem Behav. 2005 Jan;80(1):35-44. doi: 10.1016/j.pbb.2004.10.019. Epub 2004 Dec 8. Pharmacol Biochem Behav. 2005. PMID: 15652378
-
Additive antiemetic efficacy of low-doses of the cannabinoid CB(1/2) receptor agonist Δ(9)-THC with ultralow-doses of the vanilloid TRPV1 receptor agonist resiniferatoxin in the least shrew (Cryptotis parva).Eur J Pharmacol. 2014 Jan 5;722:147-55. doi: 10.1016/j.ejphar.2013.08.051. Epub 2013 Oct 22. Eur J Pharmacol. 2014. PMID: 24157976
-
The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin.Br J Pharmacol. 2008 Jan;153(2):199-215. doi: 10.1038/sj.bjp.0707442. Epub 2007 Sep 10. Br J Pharmacol. 2008. PMID: 17828291 Free PMC article. Review.
-
[Drug discrimination properties and cytotoxicity of the cannabinoid receptor ligands].Nihon Arukoru Yakubutsu Igakkai Zasshi. 2012 Jun;47(3):135-43. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2012. PMID: 22894054 Review. Japanese.
Cited by
-
Cannabis sativa: Interdisciplinary Strategies and Avenues for Medical and Commercial Progression Outside of CBD and THC.Biomedicines. 2021 Feb 26;9(3):234. doi: 10.3390/biomedicines9030234. Biomedicines. 2021. PMID: 33652704 Free PMC article. Review.
-
Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination.Medicines (Basel). 2019 Feb 23;6(1):31. doi: 10.3390/medicines6010031. Medicines (Basel). 2019. PMID: 30813390 Free PMC article. Review.
-
Interaction between non-psychotropic cannabinoids in marihuana: effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews.Psychopharmacology (Berl). 2011 Jun;215(3):505-12. doi: 10.1007/s00213-010-2157-4. Epub 2011 Jan 18. Psychopharmacology (Berl). 2011. PMID: 21243485
-
The Impact of Marijuana on Antidepressant Treatment in Adolescents: Clinical and Pharmacologic Considerations.J Pers Med. 2021 Jun 29;11(7):615. doi: 10.3390/jpm11070615. J Pers Med. 2021. PMID: 34209709 Free PMC article.
-
Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus.Br J Pharmacol. 2013 Oct;170(3):641-8. doi: 10.1111/bph.12316. Br J Pharmacol. 2013. PMID: 23889598 Free PMC article.
References
-
- Abrahamov A, Abrahamov A, Mechoulam R. An efficient new cannabinoid antiemetic in pediatric oncology. Life Sci. 1995;56:2097–2102. - PubMed
-
- Costall B, Domeney AM, Naylor RJ, Tattersal FD. Emesis induced by cisplatin in the ferret as a model for the detection of antiemetic drugs. Neuropharmacology. 1987;26:1321–1326. - PubMed
-
- Churchfield S. The Natural History of Shrews . Cornell Univ. Press Comstock Publishing Associates; Ithaca NY: 1990.
-
- Daley-Yates PT, McBrien DCH. Cisplatin metabolites in plasma, a study of their pharmacokinetics and importance in the nephrotoxic and antitumor activity of cisplatin. Biochem Pharmacol. 1984;33:3063–3070. - PubMed
-
- Darmani NA. Serotonin 5-HT3 receptor antagonists prevent cisplatin-induced emesis in Cryptotis parva: a new experimental model of emesis. J Neurol Trans. 1998;105:1143–1154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

