Cutoff and aubergine mutations result in retrotransposon upregulation and checkpoint activation in Drosophila
- PMID: 17363252
- PMCID: PMC1905832
- DOI: 10.1016/j.cub.2007.02.027
Cutoff and aubergine mutations result in retrotransposon upregulation and checkpoint activation in Drosophila
Abstract
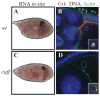
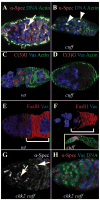
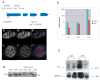
Gametogenesis is a highly regulated process in all organisms. In Drosophila, a meiotic checkpoint which monitors double-stranded DNA breaks and involves Drosophila ATR and Chk2 coordinates the meiotic cell cycle with signaling events that establish the axis of the egg and embryo. Checkpoint activity regulates translation of the transforming growth-factor-alpha-like Gurken signaling molecule which induces dorsal cell fates in the follicle cells [1-3]. We found that mutations in the Drosophila gene cutoff (cuff) affect germline cyst development and result in ventralized eggs as a result of reduced Grk protein expression. Surprisingly, cuff mutations lead to a marked increase in the transcript levels of two retrotransposable elements, Het-A and Tart. We found that small interfering RNAs against the roo element are still produced in cuff mutant ovaries. These results indicate that Cuff is involved in the rasiRNA pathway and most likely acts downstream of siRNA biogenesis. The eggshell and egg-laying defects of cuff mutants are suppressed by a mutation in chk2. We also found that mutations in aubergine (aub), another gene implicated in the rasiRNA pathway, are significantly suppressed by the chk2 mutation. Our results indicate that mutants in rasiRNA pathways lead to elevated transposition incidents in the germline, and that this elevation activates a checkpoint that causes a loss of germ cells and a reduction of Gurken protein in the remaining egg chambers.
Figures
References
-
- Nilson LA, Schupbach T. EGF receptor signaling in Drosophila oogenesis. Curr Top Dev Biol. 1999;44:203–243. - PubMed
-
- Huynh JR, St Johnston D. The origin of asymmetry: early polarisation of the Drosophila germline cyst and oocyte. Curr Biol. 2004;14:R438–449. - PubMed
-
- Ghabrial A, Schupbach T. Activation of a meiotic checkpoint regulates translation of Gurken during Drosophila oogenesis. Nat Cell Biol. 1999;1:354–357. - PubMed
-
- Abdu U, Brodsky M, Schupbach T. Activation of a meiotic checkpoint during Drosophila oogenesis regulates the translation of Gurken through Chk2/Mnk. Curr Biol. 2002;12:1645–1651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

