Charge at the lidocaine binding site residue Phe-1759 affects permeation in human cardiac voltage-gated sodium channels
- PMID: 17363383
- PMCID: PMC2075178
- DOI: 10.1113/jphysiol.2007.130161
Charge at the lidocaine binding site residue Phe-1759 affects permeation in human cardiac voltage-gated sodium channels
Abstract
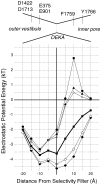
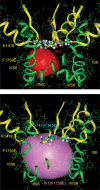
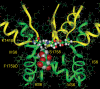
Our homology molecular model of the open/inactivated state of the Na(+) channel pore predicts, based on extensive mutagenesis data, that the local anaesthetic lidocaine docks eccentrically below the selectivity filter, such that physical occlusion is incomplete. Electrostatic field calculations suggest that the drug's positively charged amine produces an electrostatic barrier to permeation. To test the effect of charge at this pore level on permeation in hNa(V)1.5 we replaced Phe-1759 of domain IVS6, the putative binding site for lidocaine's alkylamino end, with positively and negatively charged residues as well as the neutral cysteine and alanine. These mutations eliminated use-dependent lidocaine block with no effect on tonic/rested state block. Mutant whole cell currents were kinetically similar to wild type (WT). Single channel conductance (gamma) was reduced from WT in both F1759K (by 38%) and F1759R (by 18%). The negatively charged mutant F1759E increased gamma by 14%, as expected if the charge effect were electrostatic, although F1759D was like WT. None of the charged mutations affected Na(+)/K(+) selectivity. Calculation of difference electrostatic fields in the pore model predicted that lidocaine produced the largest positive electrostatic barrier, followed by lysine and arginine, respectively. Negatively charged glutamate and aspartate both lowered the barrier, with glutamate being more effective. Experimental data were in rank order agreement with the predicted changes in the energy profile. These results demonstrate that permeation rate is sensitive to the inner pore electrostatic field, and they are consistent with creation of an electrostatic barrier to ion permeation by lidocaine's charge.
Figures
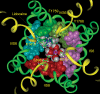




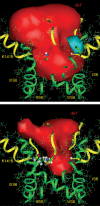
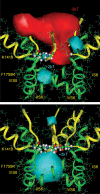
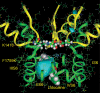
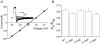
Comment in
-
Local anaesthetic block of sodium channels: raising the barrier.J Physiol. 2007 Jun 1;581(Pt 2):423. doi: 10.1113/jphysiol.2007.133637. Epub 2007 Apr 5. J Physiol. 2007. PMID: 17412761 Free PMC article. No abstract available.
References
-
- Ahern CA, Eastwood A, Lester H, Dougherty D, Horn R. Investigating cation–pi interactions in the local anesthetic block of voltage gated sodium channels. Biophys J. 2006;22a abstract.
-
- Backx PH, Yue DT, Lawrence JH, Marban E, Tomaselli GF. Molecular localization of an ion-binding site within the pore of mammalian sodium channels. Science. 1992;257:248–251. - PubMed
-
- Benitah J, Balser JR, Marban E, Tomaselli GF. Proton inhibition of sodium channels: mechanism of gating shifts and reduced conductance. J Membr Biol. 1997;155:121–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

