Circulating angiotensin II attenuates the sympathetic baroreflex by reducing the barosensitivity of medullary cardiovascular neurones in the rat
- PMID: 17363385
- PMCID: PMC2075328
- DOI: 10.1113/jphysiol.2007.128983
Circulating angiotensin II attenuates the sympathetic baroreflex by reducing the barosensitivity of medullary cardiovascular neurones in the rat
Abstract
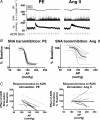
Chronic intravenous angiotensin II (Ang II) has been widely used to establish centrally mediated hypertension in experimental animals, and disruption of Ang II activity is a frontline treatment for hypertensive disease. However, the acute central actions of circulating Ang II are poorly understood. We examined the effects of intravenous pressor doses of Ang II on autonomic activity in anaesthetized rats under neuromuscular blockade, and compared baroinhibition evoked by Ang II pressor ramps to equipressor responses evoked by phenylephrine (PE). Baroinhibition of splanchnic sympathetic nerve activity was attenuated during Ang II trials compared with PE, and rats remained sensitive to electrical stimulation of the aortic depressor nerve at higher arterial pressures during Ang II trials. This was not due to a direct effect of Ang II on aortic nerve baroreceptors. In a separate series of experiments, we provide direct evidence that bulbospinal barosensitive neurones in the rostral ventrolateral medulla are differentially sensitive to pressure ramps evoked by Ang II or PE vasoconstriction. Nineteen out of 41 units were equally sensitive to increased arterial pressure evoked by Ang II or PE. In 17 of 41 units, barosensitivity was attenuated during Ang II trials, and in five of 41 cases units that had previously been barosensitive increased their firing rate during Ang II trials. These results show, for the first time, that circulating Ang II acutely modulates central cardiovascular control mechanisms. We suggest that this results from activation by Ang II of a central pathway originating at the circumventricular organs.
Figures

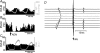

Similar articles
-
Angiotensin II and the Cardiac Parasympathetic Nervous System in Hypertension.Int J Mol Sci. 2021 Nov 14;22(22):12305. doi: 10.3390/ijms222212305. Int J Mol Sci. 2021. PMID: 34830184 Free PMC article. Review.
-
Effects of baroreceptor activation on respiratory variability in rat.Respir Physiol Neurobiol. 2009 Apr 30;166(2):80-6. doi: 10.1016/j.resp.2009.02.006. Epub 2009 Feb 24. Respir Physiol Neurobiol. 2009. PMID: 19429523 Free PMC article.
-
The rostral ventrolateral medulla mediates sympathetic baroreflex responses to intraventricular angiotensin II in rabbits.Auton Neurosci. 2003 Aug 29;107(1):20-31. doi: 10.1016/S1566-0702(03)00104-8. Auton Neurosci. 2003. PMID: 12927223
-
Activation of brain neurons by circulating angiotensin II: direct effects and baroreceptor-mediated secondary effects.Neuroscience. 1999 May;90(2):581-94. doi: 10.1016/s0306-4522(98)00572-7. Neuroscience. 1999. PMID: 10215161
-
Angiotensin and baroreflex control of the circulation.Braz J Med Biol Res. 2002 Sep;35(9):1047-59. doi: 10.1590/s0100-879x2002000900005. Epub 2002 Aug 30. Braz J Med Biol Res. 2002. PMID: 12219176 Review.
Cited by
-
Arterial baroreceptor reflex counteracts long-term blood pressure increase in the rat model of renovascular hypertension.PLoS One. 2013 Jun 6;8(6):e64788. doi: 10.1371/journal.pone.0064788. Print 2013. PLoS One. 2013. PMID: 23762254 Free PMC article.
-
Angiotensin II and the Cardiac Parasympathetic Nervous System in Hypertension.Int J Mol Sci. 2021 Nov 14;22(22):12305. doi: 10.3390/ijms222212305. Int J Mol Sci. 2021. PMID: 34830184 Free PMC article. Review.
-
Sympathoactivation and rho-kinase-dependent baroreflex function in experimental renovascular hypertension with reduced kidney mass.BMC Physiol. 2014 Jun 19;14:4. doi: 10.1186/1472-6793-14-4. BMC Physiol. 2014. PMID: 24946879 Free PMC article.
-
Is the brain the essential in hypertension?Neuroimage. 2009 Sep;47(3):914-21. doi: 10.1016/j.neuroimage.2009.04.072. Epub 2009 May 4. Neuroimage. 2009. PMID: 19410005 Free PMC article. Review.
-
Effects of baroreceptor activation on respiratory variability in rat.Respir Physiol Neurobiol. 2009 Apr 30;166(2):80-6. doi: 10.1016/j.resp.2009.02.006. Epub 2009 Feb 24. Respir Physiol Neurobiol. 2009. PMID: 19429523 Free PMC article.
References
-
- Allen AM, Moeller I, Jenkins TA, Zhuo J, Aldred GP, Chai SY, Mendelsohn FA. Angiotensin receptors in the nervous system. Brain Res Bull. 1998;47:17–28. - PubMed
-
- Badoer E, Merolli J. Neurons in the hypothalamic paraventricular nucleus that project to the rostral ventrolateral medulla are activated by haemorrhage. Brain Res. 1998;791:317–320. - PubMed
-
- Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol Regul Integr Comp Physiol. 1995;268:R625–R633. - PubMed
-
- Bishop VS, Hay M. Involvement of the area postrema in the regulation of sympathetic outflow to the cardiovascular-system. Front Neuroendocrinol. 1993;14:57–75. - PubMed
-
- Bonham AC, Hasser EM. Area postrema and aortic or vagal afferents converge to excite cells in nucleus-tractus-solitarius. Am J Physiol Heart Circ Physiol. 1993;264:H1674–H1685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

