Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits
- PMID: 17363390
- PMCID: PMC2075202
- DOI: 10.1113/jphysiol.2006.123414
Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits
Abstract
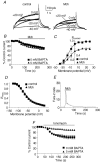
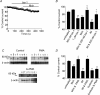
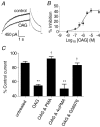
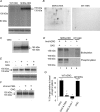
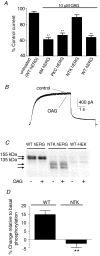
The human ether-a-go-go related gene (hERG) potassium channel is expressed in a variety of tissues including the heart, neurons and some cancer cells. hERG channels are modulated by several intracellular signalling pathways and these provide important mechanisms for regulating cellular excitability. In this study, we investigated muscarinic modulation of hERG currents and direct phosphorylation of channel subunits expressed in HEK-293 cells at physiologically relevant temperatures by protein kinase C (PKC). Activation of G(alpha q/11)-coupled M(3)-muscarinic receptors with methacholine, reduced current amplitudes at all potentials with minor effects on the voltage dependence of activation and inactivation. The response to methacholine was insensitive to intracellular BAPTA, but was attenuated by either acute inhibition of PKC with 300 nm bisindolylmaleimide-1 (bis-1) or chronic down-regulation of PKC isoforms by 24 h pretreatment of cells with phorbol 12-myristate 13-acetate (PMA). Stimulation of PKC with 1-oleoyl 2-acetylglycerol (OAG), an analogue of diacylglycerol (DAG), mimicked the actions of muscarinic receptor stimulation. Direct phosphorylation of hERG was measured by [(32)P]orthophosphate labelling of immunoprecipitated protein with an anti-hERG antibody. Basal phosphorylation was high in unstimulated cells and further increased by OAG. The OAG dependent increase was abolished by bis-1 and down-regulation of PKC, but basal levels of phosphorylation were unchanged. Deletion of the amino-terminus of hERG prevented both the modulation of channel activity and the increase of phosphorylation by OAG. Our results are consistent with calcium and/or DAG sensitive isotypes of PKC modulating hERG currents through a mechanism that involves direct phosphorylation of sites on the amino terminus of hERG.
Figures
Similar articles
-
Modulation of HERG K+ channels by chronic exposure to activators and inhibitors of PKA and PKC: actions independent of PKA and PKC phosphorylation.Cell Physiol Biochem. 2013;32(6):1830-44. doi: 10.1159/000356616. Epub 2013 Dec 16. Cell Physiol Biochem. 2013. PMID: 24356123
-
Regulation of HERG potassium channel activation by protein kinase C independent of direct phosphorylation of the channel protein.Cardiovasc Res. 2003 Jul 1;59(1):14-26. doi: 10.1016/s0008-6363(03)00386-9. Cardiovasc Res. 2003. PMID: 12829172
-
Modulation of rat erg1, erg2, erg3 and HERG K+ currents by thyrotropin-releasing hormone in anterior pituitary cells via the native signal cascade.J Physiol. 2001 Apr 1;532(Pt 1):143-63. doi: 10.1111/j.1469-7793.2001.0143g.x. J Physiol. 2001. PMID: 11283231 Free PMC article.
-
Inhibition of the rapid component of the delayed rectifier potassium current in ventricular myocytes by angiotensin II via the AT1 receptor.Br J Pharmacol. 2008 May;154(2):429-39. doi: 10.1038/bjp.2008.95. Epub 2008 Apr 14. Br J Pharmacol. 2008. PMID: 18414380 Free PMC article.
-
Effects of the PKC inhibitors chelerythrine and bisindolylmaleimide I (GF 109203X) on delayed rectifier K+ currents.Naunyn Schmiedebergs Arch Pharmacol. 2011 Feb;383(2):141-8. doi: 10.1007/s00210-010-0584-8. Epub 2010 Dec 1. Naunyn Schmiedebergs Arch Pharmacol. 2011. PMID: 21120453
Cited by
-
Phosphatidylinositol 4,5-bisphosphate interactions with the HERG K(+) channel.Pflugers Arch. 2007 Oct;455(1):105-13. doi: 10.1007/s00424-007-0292-5. Epub 2007 Jul 11. Pflugers Arch. 2007. PMID: 17622552 Review.
-
Role of ERG1 isoforms in modulation of ERG1 channel trafficking and function.Pflugers Arch. 2010 Oct;460(5):803-12. doi: 10.1007/s00424-010-0855-8. Epub 2010 Jun 24. Pflugers Arch. 2010. PMID: 20574821 Review.
-
Cytoplasmic domains and voltage-dependent potassium channel gating.Front Pharmacol. 2012 Mar 23;3:49. doi: 10.3389/fphar.2012.00049. eCollection 2012. Front Pharmacol. 2012. PMID: 22470342 Free PMC article.
-
Modulation of Ether-à-Go-Go Related Gene (ERG) Current Governs Intrinsic Persistent Activity in Rodent Neocortical Pyramidal Cells.J Neurosci. 2018 Jan 10;38(2):423-440. doi: 10.1523/JNEUROSCI.1774-17.2017. Epub 2017 Nov 24. J Neurosci. 2018. PMID: 29175952 Free PMC article.
-
Obesity Arrhythmias: Role of IL-6 Trans-Signaling.Int J Mol Sci. 2024 Aug 1;25(15):8407. doi: 10.3390/ijms25158407. Int J Mol Sci. 2024. PMID: 39125976 Free PMC article. Review.
References
-
- Arcangeli A. Expression and role of hERG channels in cancer cells. In: Chadwick DJ, Goode J, editors. The hERG Cardiac Potassium Channel: Structure, Function and Long QT Syndrome. Chichester, UK: John Wiley & Sons Ltd; 2005. pp. 225–232. Novartis Foundation Symposium 266.
-
- Bauer CK, Wulfsen I, Schafer R, Glassmeier G, Wimmers S, Flitsch J, Ludecke DK, Schwarz JR. HERG K+ currents in human prolactin-secreting adenoma cells. Pflugers Arch. 2003;445:589–600. - PubMed
-
- Boyle WJ, Van Der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. - PubMed
-
- Budd D, Challiss R, Young K, Tobin A. Cross talk between M3-muscarinic and β2-adrenergic receptors at the level of receptor phosphorylationand desensitization. Mol Pharmacol. 1999;56:813–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

