Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial
- PMID: 17363401
- PMCID: PMC1955127
- DOI: 10.1136/ard.2006.065128
Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial
Abstract
Objective: To compare epicutaneous ketoprofen in Transfersome (ultra-deformable vesicles, IDEA-033) versus oral celecoxib and placebo for relief of signs and symptoms in knee osteoarthritis.
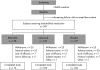
Methods: This was a multicentre, randomised, double-blind, controlled trial; 397 patients with knee osteoarthritis participated and 324 completed the trial. They were randomly assigned 110 mg epicutaneous ketoprofen in 4.8 g Transfersome plus oral placebo (n = 138), 100 mg oral celecoxib plus placebo gel (n = 132), or both placebo formulations (n = 127) twice daily for 6 weeks. Primary efficacy outcome measures were the changes from baseline to end of the study on the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index pain subscale, physical function subscale and patient global assessment (PGA) of response.
Results: The mean WOMAC pain subscale scores in the intent to treat population were reduced by 18.2 (95% confidence interval -22.1 to -14.3), 20.3 (-24.3 to -16.2) and 9.9 (-13.9 to -5.8) in the IDEA-033, celecoxib and placebo groups, respectively, and the physical function subscale score by 14.6 (-18.1 to -11.0), 16.6 (-20.2 to -13.0) and 10.2 (-13.8 to -6.6), respectively. The mean PGA of response scores were 1.8 (1.6 to 2.1), 1.7 (1.5 to 1.9) and 1.3 (1.1 to 1.5), respectively. The differences in change between IDEA-033 and placebo were statistically significant for pain subscale (p<0.01) and PGA of response (p<0.01). Gastrointestinal adverse events for IDEA-033 were similar to placebo.
Conclusion: IDEA-033 is superior to placebo and comparable with celecoxib in relieving pain associated with an acute flare of knee osteoarthritis.
Conflict of interest statement
IDEA AG (Germany) and McNeill Consumer & Specialty Pharmaceuticals (USA) sponsored the study and carried out on‐site monitoring of all participants. The sponsors had an opportunity to comment on the manuscript before submission, but the final version was the sole responsibility of the authors.
References
-
- Scott J C, Hochbert M C. Arthritic and other musculosceletal diseases. In: Brownson RC, Remington PL, Davis JR, eds. Chronic disease: epidemiology and control Washington: American Public Health Association, 1993
-
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000431905–1915. - PubMed
-
- Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma J W.et al EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 200059936–944. - PMC - PubMed
-
- Mazieres B, Bannwarth B, Dougados M, Lequesne M. EULAR recommendations for the management of knee osteoarthritis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials. Joint Bone Spine 200168231–240. - PubMed
-
- Roddy E, Doherty M. Guidelines for management of osteoarthritis published by the American College of Rheumatology and the European League Against Rheumatism: why are they so different? Rheum Dis Clin North Am 200329717–731. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical