Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer
- PMID: 17363532
- PMCID: PMC3203693
- DOI: 10.1158/1078-0432.CCR-06-1863
Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer
Abstract
Purpose: To define the maximum tolerated dose (MTD), toxicities, and pharmacokinetics of 17-allylamino-17-demethoxygeldanamycin (17-AAG) when administered using continuous and intermittent dosing schedules.
Experimental design: Patients with progressive solid tumor malignancies were treated with 17-AAG using an accelerated titration dose escalation schema. The starting dose and schedule were 5 mg/m(2) daily for 5 days with cycles repeated every 21 days. Dosing modifications based on safety, pharmacodynamic modeling, and clinical outcomes led to the evaluation of the following schedules: daily x 3 repeated every 14 days; twice weekly (days 1, 4, 8, and 11) for 2 weeks every 3 weeks; and twice weekly (days 1 and 4) without interruption. During cycle 1, blood was collected for pharmacokinetic and pharmacodynamic studies.
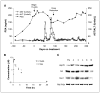
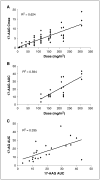
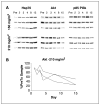
Results: Fifty-four eligible patients were treated. The MTD was schedule dependent: 56 mg/m(2) on the daily x 5 schedule; 112 mg/m(2) on the daily x 3 schedule; and 220 mg/m(2) on the days 1, 4, 8, and 11 every-21-day schedule. Continuous twice-weekly dosing was deemed too toxic because of delayed hepatotoxicity. Hepatic toxicity was also dose limiting with the daily x 5 schedule. Other common toxicities encountered were fatigue, myalgias, and nausea. This latter adverse effect may have been attributable, in part, to the DMSO-based formulation. Concentrations of 17-AAG above those required for activity in preclinical models could be safely achieved in plasma. Induction of a heat shock response and down-regulation of Akt and Raf-1 were observed in biomarker studies.
Conclusion: The MTD and toxicity profile of 17-AAG were schedule dependent. Intermittent dosing schedules were less toxic and are recommended for future phase II studies.
Figures
Comment in
-
Targeting the molecular chaperone heat shock protein 90 provides a multifaceted effect on diverse cell signaling pathways of cancer cells.Clin Cancer Res. 2007 Mar 15;13(6):1625-9. doi: 10.1158/1078-0432.CCR-06-2966. Clin Cancer Res. 2007. PMID: 17363512 Review. No abstract available.
Similar articles
-
Phase I and pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin in adult patients with refractory advanced cancers.Clin Cancer Res. 2007 Mar 15;13(6):1769-74. doi: 10.1158/1078-0432.CCR-06-2233. Clin Cancer Res. 2007. PMID: 17363531 Clinical Trial.
-
Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies.Eur J Cancer. 2010 Jan;46(2):340-7. doi: 10.1016/j.ejca.2009.10.026. Epub 2009 Nov 27. Eur J Cancer. 2010. PMID: 19945858 Free PMC article. Clinical Trial.
-
Phase I pharmacokinetic and pharmacodynamic study of 17-N-allylamino-17-demethoxygeldanamycin in pediatric patients with recurrent or refractory solid tumors: a pediatric oncology experimental therapeutics investigators consortium study.Clin Cancer Res. 2007 Mar 15;13(6):1783-8. doi: 10.1158/1078-0432.CCR-06-1892. Clin Cancer Res. 2007. PMID: 17363533 Clinical Trial.
-
A phase I trial of docetaxel and pulse-dose 17-allylamino-17-demethoxygeldanamycin in adult patients with solid tumors.Cancer Chemother Pharmacol. 2012 Apr;69(4):1089-97. doi: 10.1007/s00280-011-1789-3. Epub 2011 Nov 29. Cancer Chemother Pharmacol. 2012. PMID: 22124669 Free PMC article. Clinical Trial.
-
Phase I. Trial of irinotecan plus carboplatin in two dose schedules.Oncology (Williston Park). 2003 May;17(5 Suppl 5):36-40. Oncology (Williston Park). 2003. PMID: 12800605 Review.
Cited by
-
Overexpression of DJ-1 and HSP90α, and loss of PTEN associated with invasive urothelial carcinoma of urinary bladder: Possible prognostic markers.Oncol Lett. 2012 Mar;3(3):507-512. doi: 10.3892/ol.2011.522. Epub 2011 Dec 13. Oncol Lett. 2012. PMID: 22740940 Free PMC article.
-
HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans.J Clin Invest. 2010 Oct;120(10):3578-93. doi: 10.1172/JCI42442. Epub 2010 Sep 13. J Clin Invest. 2010. PMID: 20852385 Free PMC article.
-
Heat shock protein 90 inhibitors suppress aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and DNA adduct formation.Cancer Prev Res (Phila). 2008 Nov;1(6):485-93. doi: 10.1158/1940-6207.CAPR-08-0149. Cancer Prev Res (Phila). 2008. Retraction in: Cancer Prev Res (Phila). 2022 Jun 2;15(6):415. doi: 10.1158/1940-6207.CAPR-22-0200. PMID: 19138996 Free PMC article. Retracted.
-
Molecularly targeted agents as radiosensitizers in cancer therapy--focus on prostate cancer.Int J Mol Sci. 2013 Jul 16;14(7):14800-32. doi: 10.3390/ijms140714800. Int J Mol Sci. 2013. PMID: 23863691 Free PMC article. Review.
-
Nanocarrier-mediated immunogenic chemotherapy for triple negative breast cancer.J Control Release. 2020 Jul 10;323:431-441. doi: 10.1016/j.jconrel.2020.04.040. Epub 2020 Apr 30. J Control Release. 2020. PMID: 32360890 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

