Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling
- PMID: 17363559
- PMCID: PMC2100408
- DOI: 10.1158/0008-5472.CAN-06-3977
Extrathymic generation of tumor-specific T cells from genetically engineered human hematopoietic stem cells via Notch signaling
Abstract
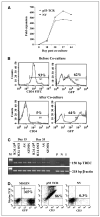
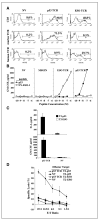
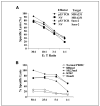
Adoptive cell transfer (ACT) of tumor-reactive lymphocytes has been shown to be an effective treatment for cancer patients. Studies in murine models of ACT indicated that antitumor efficacy of adoptively transferred T cells is dependent on the differentiation status of the cells, with lymphocyte differentiation inversely correlated with in vivo antitumor effectiveness. T-cell in vitro development technologies provide a new opportunity to generate naive T cells for the purpose of ACT. In this study, we genetically modified human umbilical cord blood-derived hematopoietic stem cells (HSCs) to express tumor antigen-specific T-cell receptor (TCR) genes and generated T lymphocytes by coculture with a murine cell line expressing Notch-1 ligand, Delta-like-1 (OP9-DL1). Input HSCs were differentiated into T cells as evidenced by the expression of T-cell markers, such as CD7, CD1a, CD4, CD8, and CD3, and by detection of TCR excision circles. We found that such in vitro differentiated T cells expressed the TCR and showed HLA-A2-restricted, specific recognition and killing of tumor antigen peptide-pulsed antigen-presenting cells but manifested additional natural killer cell-like killing of tumor cell lines. The genetic manipulation of HSCs has broad implications for ACT of cancer.
Figures

Similar articles
-
Manipulation of human early T lymphopoiesis by coculture on human bone marrow stromal cells: potential utility for adoptive immunotherapy.Exp Hematol. 2013 Apr;41(4):367-76.e1. doi: 10.1016/j.exphem.2012.12.001. Epub 2012 Dec 17. Exp Hematol. 2013. PMID: 23257689
-
Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro.Blood. 2005 Feb 15;105(4):1431-9. doi: 10.1182/blood-2004-04-1293. Epub 2004 Oct 19. Blood. 2005. PMID: 15494433
-
Generation of functional, antigen-specific CD8+ human T cells from cord blood stem cells using exogenous Notch and tetramer-TCR signaling.Stem Cells. 2014 Jan;32(1):93-104. doi: 10.1002/stem.1512. Stem Cells. 2014. PMID: 23939944 Free PMC article.
-
Synergy between the pre-T cell receptor and Notch: cementing the alphabeta lineage choice.J Exp Med. 2006 Oct 2;203(10):2233-7. doi: 10.1084/jem.20060998. Epub 2006 Sep 25. J Exp Med. 2006. PMID: 17000868 Free PMC article. Review.
-
Generation of immunocompetent T cells from embryonic stem cells.Methods Mol Biol. 2007;380:73-81. doi: 10.1007/978-1-59745-395-0_5. Methods Mol Biol. 2007. PMID: 17876088 Review.
Cited by
-
Applications of molecular engineering in T-cell-based immunotherapies.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019 Sep;11(5):e1557. doi: 10.1002/wnan.1557. Epub 2019 Apr 10. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019. PMID: 30972976 Free PMC article. Review.
-
Melanoma Immunotherapy in Mice Using Genetically Engineered Pluripotent Stem Cells.Cell Transplant. 2016;25(5):811-27. doi: 10.3727/096368916X690467. Epub 2016 Jan 15. Cell Transplant. 2016. PMID: 26777320 Free PMC article.
-
In vitro differentiation of adult bone marrow progenitors into antigen-specific CD4 helper T cells using engineered stromal cells expressing a notch ligand and a major histocompatibility complex class II protein.Stem Cells Dev. 2009 Mar;18(2):235-45. doi: 10.1089/scd.2008.0021. Stem Cells Dev. 2009. PMID: 18680390 Free PMC article.
-
Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells.J Immunother. 2010 Jan;33(1):1-7. doi: 10.1097/CJI.0b013e3181b88ffc. J Immunother. 2010. PMID: 19952961 Free PMC article.
-
Specific Targeting of Notch Ligand-Receptor Interactions to Modulate Immune Responses: A Review of Clinical and Preclinical Findings.Front Immunol. 2020 Aug 14;11:1958. doi: 10.3389/fimmu.2020.01958. eCollection 2020. Front Immunol. 2020. PMID: 32922403 Free PMC article. Review.
References
-
- Stanislawski T, Voss RH, Lotz C, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2:962–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

