Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure
- PMID: 17363700
- PMCID: PMC2386857
- DOI: 10.1161/01.RES.0000264081.78659.45
Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure
Abstract
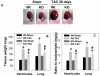
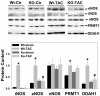
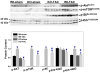
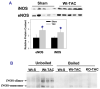
Inducible nitric oxide synthase (iNOS) protein is expressed in cardiac myocytes of patients and experimental animals with congestive heart failure (CHF). Here we show that iNOS expression plays a role in pressure overload-induced myocardial chamber dilation and hypertrophy. In wild-type mice, chronic transverse aortic constriction (TAC) resulted in myocardial iNOS expression, cardiac hypertrophy, ventricular dilation and dysfunction, and fibrosis, whereas iNOS-deficient mice displayed much less hypertrophy, dilation, fibrosis, and dysfunction. Consistent with these findings, TAC resulted in marked increases of myocardial atrial natriuretic peptide 4-hydroxy-2-nonenal (a marker of lipid peroxidation) and nitrotyrosine (a marker for peroxynitrite) in wild-type mice but not in iNOS-deficient mice. In response to TAC, myocardial endothelial NO synthase and iNOS was expressed as both monomer and dimer in wild-type mice, and this was associated with increased reactive oxygen species production, suggesting that iNOS monomer was a source for the increased oxidative stress. Moreover, systolic overload-induced Akt, mammalian target of rapamycin, and ribosomal protein S6 activation was significantly attenuated in iNOS-deficient mice. Furthermore, selective iNOS inhibition with 1400W (6 mg/kg per hour) significantly attenuated TAC induced myocardial hypertrophy and pulmonary congestion. These data implicate iNOS in the maladaptative response to systolic overload and suggest that selective iNOS inhibition or attenuation of iNOS monomer content might be effective for treatment of systolic overload-induced cardiac dysfunction.
Figures







Similar articles
-
Ecto-5'-nucleotidase deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction.Hypertension. 2008 Jun;51(6):1557-64. doi: 10.1161/HYPERTENSIONAHA.108.110833. Epub 2008 Apr 7. Hypertension. 2008. PMID: 18391093 Free PMC article.
-
Celiprolol, a vasodilatory beta-blocker, inhibits pressure overload-induced cardiac hypertrophy and prevents the transition to heart failure via nitric oxide-dependent mechanisms in mice.Circulation. 2004 Aug 10;110(6):692-9. doi: 10.1161/01.CIR.0000137831.08683.E1. Epub 2004 Jul 19. Circulation. 2004. PMID: 15262839
-
Role of myocardial inducible nitric oxide synthase in contractile dysfunction and beta-adrenergic hyporesponsiveness in rats with experimental volume-overload heart failure.Circulation. 2002 Jan 15;105(2):236-43. doi: 10.1161/hc0202.102015. Circulation. 2002. PMID: 11790707
-
AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice.Hypertension. 2008 Nov;52(5):918-24. doi: 10.1161/HYPERTENSIONAHA.108.114702. Epub 2008 Oct 6. Hypertension. 2008. PMID: 18838626 Free PMC article.
-
Statins and the myocardium.Semin Vasc Med. 2004 Nov;4(4):377-84. doi: 10.1055/s-2004-869594. Semin Vasc Med. 2004. PMID: 15861318 Free PMC article. Review.
Cited by
-
Altered Nitric Oxide System in Cardiovascular and Renal Diseases.Chonnam Med J. 2016 May;52(2):81-90. doi: 10.4068/cmj.2016.52.2.81. Epub 2016 May 20. Chonnam Med J. 2016. PMID: 27231671 Free PMC article. Review.
-
Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration.Nat Chem Biol. 2012 Aug;8(8):714-24. doi: 10.1038/nchembio.1018. Epub 2012 Jul 1. Nat Chem Biol. 2012. PMID: 22772154 Free PMC article.
-
iNOS expressing macrophages co-localize with nitrotyrosine staining after myocardial infarction in humans.Front Cardiovasc Med. 2023 Mar 30;10:1104019. doi: 10.3389/fcvm.2023.1104019. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37063955 Free PMC article.
-
Is Inducible Nitric Oxide Synthase (iNOS) Promising as a New Target Against Pulmonary Hypertension?Antioxidants (Basel). 2025 Mar 21;14(4):377. doi: 10.3390/antiox14040377. Antioxidants (Basel). 2025. PMID: 40298665 Free PMC article. Review.
-
Vascular endothelial-specific dimethylarginine dimethylaminohydrolase-1-deficient mice reveal that vascular endothelium plays an important role in removing asymmetric dimethylarginine.Circulation. 2009 Dec 1;120(22):2222-9. doi: 10.1161/CIRCULATIONAHA.108.819912. Epub 2009 Nov 16. Circulation. 2009. PMID: 19917889 Free PMC article.
References
-
- Haywood GA, Tsao PS, von der Leyen HE, Mann MJ, Keeling PJ, Trindade PT, Lewis NP, Byrne CD, Rickenbacher PR, Bishopric NH, Cooke JP, McKenna WJ, Fowler MB. Expression of inducible nitric oxide synthase in human heart failure. Circulation. 1996;93:1087–1094. - PubMed
-
- Habib FM, Springall DR, Davies GJ, Oakley CM, Yacoub MH, Polak JM. Tumour necrosis factor and in dilated cardiomyopathy. Lancet. 1996;347:1151–1155. - PubMed
-
- Chen Y, Traverse JH, Du R, Hou M, Bache RJ. Nitric oxide modulates myocardial oxygen consumption in the failing heart. Circulation. 2002;106:273–9. - PubMed
-
- Nadruz W, Jr, Lagosta VJ, Moreno H, Jr, Coelho OR, Franchini KG. Simvastatin prevents load-induced protein tyrosine nitration in overloaded hearts. Hypertension. 2004;43:1060–6. - PubMed
-
- Liu YH, Carretero OA, Cingolani OH, Liao TD, Sun Y, Xu J, Li LY, Pagano PJ, Yang JJ, Yang XP. Role of Inducible Nitric Oxide Synthase in Cardiac Function and Remodeling in Mice with Heart Failure Due to Myocardial Infarction. Am J Physiol Heart Circ Physiol. 2005;289:H2616–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

