CD13/APN regulates endothelial invasion and filopodia formation
- PMID: 17363739
- PMCID: PMC1896108
- DOI: 10.1182/blood-2006-02-002931
CD13/APN regulates endothelial invasion and filopodia formation
Abstract
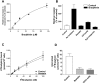
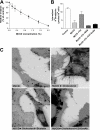
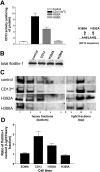
CD13/aminopeptidase N is a transmembrane peptidase that is induced in the vasculature of solid tumors and is a potent angiogenic regulator. Here, we demonstrate that CD13 controls endothelial cell invasion in response to the serum peptide bradykinin by facilitating signal transduction at the level of the plasma membrane. Inhibition of CD13 abrogates bradykinin B(2) receptor internalization, leading to the attenuation of downstream events such as bradykinin-induced activation of Cdc42 and filopodia formation, and thus affects endothelial cell motility. Investigation into mechanisms underlying this block led us to focus on B(2)R internalization via membrane-dependent mechanisms. Membrane disruption by depletion of cholesterol or trypsinization halts B(2)R internalization, invasion, and filopodia formation, which can be recovered with addition of cholesterol. However, this functional recovery is severely impaired in the presence of CD13 antagonists, and the distribution of membrane proteins is disordered in treated cells, suggesting a role for CD13 in plasma membrane protein organization. Finally, exogenous expression of wild-type but not mutant CD13 further alters protein distribution, suggesting peptidase activity is required for CD13's regulatory activity. Therefore, CD13 functions as a novel modulator of signal transduction and cell motility via its influence on specific plasma membrane organization, thus regulating angiogenesis.
Figures




References
-
- Blaukat A. Structure and signalling pathways of kinin receptors. Andrologia. 2003;35:17–23. - PubMed
-
- Prado GN, Taylor L, Zhou X, Ricupero D, Mierke DF, Polgar P. Mechanisms regulating the expression, self-maintenance, and signaling-function of the bradykinin B2 and B1 receptors. J Cell Physiol. 2002;193:275–286. - PubMed
-
- Hayashi I, Amano H, Yoshida S, et al. Suppressed angiogenesis in kininogen-deficiencies. Lab Invest. 2002;82:871–880. - PubMed
-
- Colman RW, Pixley RA, Sainz IM, et al. Inhibition of angiogenesis by antibody blocking the action of proangiogenic high-molecular-weight kininogen. J Thromb Haemost. 2003;1:164–170. - PubMed
-
- Song JS, Sainz IM, Cosenza SC, et al. Inhibition of tumor angiogenesis in vivo by a monoclonal antibody targeted to domain 5 of high molecular weight kininogen. Blood. 2004;104:2065–2072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

