Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-gamma receptor-deficient mice
- PMID: 17363768
- PMCID: PMC1899276
- DOI: 10.1164/rccm.200610-1426OC
Control of virus reactivation arrests pulmonary herpesvirus-induced fibrosis in IFN-gamma receptor-deficient mice
Abstract
Rationale: Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrotic lung disorder of unknown cause. Several studies suggest an association between Epstein-Barr virus pulmonary infection and the development of IPF.
Objectives: To determine whether reduction of gamma-herpesvirus reactivation from latency would alter progressive lung fibrogenesis in an animal model of virus-induced pulmonary fibrosis.
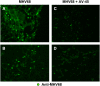
Methods: IFN-gamma receptor-deficient (IFN-gammaR(-/-)) mice infected intranasally with murine gamma-herpesvirus 68 (MHV68) develop lung fibrosis that progresses for up to at least 180 days after initial infection. Viral replication during the chronic phase of infection was controlled by two methods: the administration of cidofovir, an antiviral drug effective at clearing lytic but not latent virus, and by using a mutant gamma-herpesvirus defective in virus reactivation from latency.
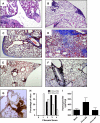
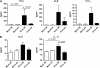
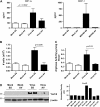
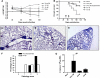
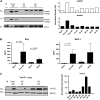
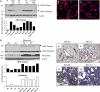
Measurements and main results: Ten percent of the asymptomatic MHV68-infected animals that received antiviral treatment beginning on Day 45 postinfection had severe pulmonary fibrosis compared with 40% of the control saline-treated animals. Absence of severe fibrosis was also observed in IFN-gammaR(-/-) mice infected with the defective reactivation mutant MHV68 v-cyclin stop. Decreased fibrosis was associated with lower levels of transforming growth factor-beta, vascular endothelial growth factor, and markers of macrophage alternative activation. When antiviral treatment was administered on Day 60 in symptomatic animals, survival improved from 20 to 80% compared with untreated symptomatic animals, but lung fibrosis persisted in 60% of the mice.
Conclusions: MHV68-induced fibrosis is a result of viral lytic replication during chronic lung herpesvirus infection in mice. We speculate that antiviral therapy might help to control lung fibrosis in humans with IPF and associated herpesvirus infection.
Figures


Comment in
-
Taking the "idio" out of idiopathic pulmonary fibrosis: a call to arms.Am J Respir Crit Care Med. 2007 Jun 1;175(11):1101-2. doi: 10.1164/rccm.200704-547ED. Am J Respir Crit Care Med. 2007. PMID: 17519345 No abstract available.
Similar articles
-
Lung infection with gamma-herpesvirus induces progressive pulmonary fibrosis in Th2-biased mice.Am J Physiol Lung Cell Mol Physiol. 2005 Nov;289(5):L711-21. doi: 10.1152/ajplung.00007.2005. Epub 2005 Feb 25. Am J Physiol Lung Cell Mol Physiol. 2005. PMID: 15734789
-
Inhibition of NF-kappaB signaling reduces virus load and gammaherpesvirus-induced pulmonary fibrosis.Am J Pathol. 2010 Aug;177(2):608-21. doi: 10.2353/ajpath.2010.091122. Epub 2010 Jun 21. Am J Pathol. 2010. PMID: 20566741 Free PMC article.
-
Activation of alveolar macrophages via the alternative pathway in herpesvirus-induced lung fibrosis.Am J Respir Cell Mol Biol. 2006 Oct;35(4):466-73. doi: 10.1165/rcmb.2006-0121OC. Epub 2006 May 18. Am J Respir Cell Mol Biol. 2006. PMID: 16709958 Free PMC article.
-
Natural history of murine gamma-herpesvirus infection.Philos Trans R Soc Lond B Biol Sci. 2001 Apr 29;356(1408):569-79. doi: 10.1098/rstb.2000.0779. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11313012 Free PMC article. Review.
-
Murine Gammaherpesvirus 68: A Small Animal Model for Gammaherpesvirus-Associated Diseases.Adv Exp Med Biol. 2017;1018:225-236. doi: 10.1007/978-981-10-5765-6_14. Adv Exp Med Biol. 2017. PMID: 29052141 Review.
Cited by
-
Right place, right time: the evolving role of herpesvirus infection as a "second hit" in idiopathic pulmonary fibrosis.Am J Physiol Lung Cell Mol Physiol. 2012 Mar 1;302(5):L441-4. doi: 10.1152/ajplung.00335.2011. Epub 2011 Dec 16. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22180659 Free PMC article.
-
A Conserved Gammaherpesvirus Cyclin Specifically Bypasses Host p18(INK4c) To Promote Reactivation from Latency.J Virol. 2015 Nov;89(21):10821-31. doi: 10.1128/JVI.00891-15. Epub 2015 Aug 19. J Virol. 2015. PMID: 26292318 Free PMC article.
-
CD8+ T Cell Response to Gammaherpesvirus Infection Mediates Inflammation and Fibrosis in Interferon Gamma Receptor-Deficient Mice.PLoS One. 2015 Aug 28;10(8):e0135719. doi: 10.1371/journal.pone.0135719. eCollection 2015. PLoS One. 2015. PMID: 26317335 Free PMC article.
-
Ataxia telangiectasia mutated kinase controls chronic gammaherpesvirus infection.J Virol. 2012 Dec;86(23):12826-37. doi: 10.1128/JVI.00917-12. Epub 2012 Sep 19. J Virol. 2012. PMID: 22993144 Free PMC article.
-
γ-Herpes virus-68, but not Pseudomonas aeruginosa or influenza A (H1N1), exacerbates established murine lung fibrosis.Am J Physiol Lung Cell Mol Physiol. 2014 Aug 1;307(3):L219-30. doi: 10.1152/ajplung.00300.2013. Epub 2014 May 30. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24879051 Free PMC article.
References
-
- American Thoracic Society; European Respiratory Society.Idiopathic pulmonary fibrosis: diagnosis and treatment [International Consensus Statement]. Am J Respir Crit Care Med 2000;161:646–664. - PubMed
-
- Selman M, Pardo A. The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S93–S97. - PubMed
-
- Perez A, Rogers RM, Dauber JH. The prognosis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S19–S26. - PubMed
-
- Stewart JP, Egan JJ, Ross AJ, Kelly BG, Lok SS, Hasleton PS, Woodcock AA. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1999;159:1336–1341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

