Phosphorylation and ubiquitination of the IkappaB kinase complex by two distinct signaling pathways
- PMID: 17363905
- PMCID: PMC1847656
- DOI: 10.1038/sj.emboj.7601622
Phosphorylation and ubiquitination of the IkappaB kinase complex by two distinct signaling pathways
Abstract
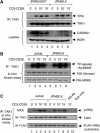
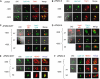
The IkappaB kinase (IKK) complex serves as the master regulator for the activation of NF-kappaB by various stimuli. It contains two catalytic subunits, IKKalpha and IKKbeta, and a regulatory subunit, IKKgamma/NEMO. The activation of IKK complex is dependent on the phosphorylation of IKKalpha/beta at its activation loop and the K63-linked ubiquitination of NEMO. However, the molecular mechanism by which these inducible modifications occur remains undefined. Here, we demonstrate that CARMA1, a key scaffold molecule, is essential to regulate NEMO ubiquitination upon T-cell receptor (TCR) stimulation. However, the phosphorylation of IKKalpha/beta activation loop is independent of CARMA1 or NEMO ubiquitination. Further, we provide evidence that TAK1 is activated and recruited to the synapses in a CARMA1-independent manner and mediate IKKalpha/beta phosphorylation. Thus, our study provides the biochemical and genetic evidence that phosphorylation of IKKalpha/beta and ubiquitination of NEMO are regulated by two distinct pathways upon TCR stimulation.
Figures






Similar articles
-
PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation.Science. 2005 Apr 1;308(5718):114-8. doi: 10.1126/science.1107107. Science. 2005. PMID: 15802604
-
NF-kappaB activation in T cells requires discrete control of IkappaB kinase alpha/beta (IKKalpha/beta) phosphorylation and IKKgamma ubiquitination by the ADAP adapter protein.J Biol Chem. 2010 Apr 9;285(15):11100-5. doi: 10.1074/jbc.M109.068999. Epub 2010 Feb 17. J Biol Chem. 2010. PMID: 20164171 Free PMC article.
-
T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b.Mol Cell Biol. 2008 Apr;28(7):2470-80. doi: 10.1128/MCB.01505-07. Epub 2008 Jan 28. Mol Cell Biol. 2008. PMID: 18227156 Free PMC article.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
The IκB kinase complex in NF-κB regulation and beyond.EMBO Rep. 2014 Jan;15(1):46-61. doi: 10.1002/embr.201337983. Epub 2013 Dec 27. EMBO Rep. 2014. PMID: 24375677 Free PMC article. Review.
Cited by
-
K63-linked ubiquitination in kinase activation and cancer.Front Oncol. 2012 Jan 31;2:5. doi: 10.3389/fonc.2012.00005. eCollection 2012. Front Oncol. 2012. PMID: 22649774 Free PMC article.
-
PKCθ synergizes with TLR-dependent TRAF6 signaling pathway to upregulate MUC5AC mucin via CARMA1.PLoS One. 2012;7(1):e31049. doi: 10.1371/journal.pone.0031049. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22303480 Free PMC article.
-
Malt1 ubiquitination triggers NF-kappaB signaling upon T-cell activation.EMBO J. 2007 Nov 14;26(22):4634-45. doi: 10.1038/sj.emboj.7601897. Epub 2007 Oct 18. EMBO J. 2007. PMID: 17948050 Free PMC article.
-
Mechanisms of Regulated and Dysregulated CARD11 Signaling in Adaptive Immunity and Disease.Front Immunol. 2018 Sep 19;9:2105. doi: 10.3389/fimmu.2018.02105. eCollection 2018. Front Immunol. 2018. PMID: 30283447 Free PMC article. Review.
-
Polyubiquitination events mediate polymethylmethacrylate (PMMA) particle activation of NF-kappaB pathway.J Biol Chem. 2011 Jul 8;286(27):23735-41. doi: 10.1074/jbc.M111.223669. Epub 2011 May 12. J Biol Chem. 2011. PMID: 21566132 Free PMC article.
References
-
- Baldwin AS Jr (1996) The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 14: 649–683 - PubMed
-
- Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML (2001) The immunological synapse. Annu Rev Immunol 19: 375–396 - PubMed
-
- Che T, You Y, Wang D, Tanner MJ, Dixit VM, Lin X (2004) MALT1/paracaspase is a signaling component downstream of CARMA1 and mediates T-cell receptor-induced NF-kappaB activation. J Biol Chem 279: 15870–15876 - PubMed
-
- Chen ZJ, Bhoj V, Seth RB (2006) Ubiquitin, TAK1 and IKK: is there a connection? Cell Death Differ 13: 687–692 - PubMed
-
- Egawa T, Albrecht B, Favier B, Sunshine MJ, Mirchandani K, O'Brien W, Thome M, Littman DR (2003) Requirement for CARMA1 in antigen receptor-induced NF-kappa B activation and lymphocyte proliferation. Curr Biol 13: 1252–1258 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

