Scientific barriers to developing vaccines against avian influenza viruses
- PMID: 17363960
- PMCID: PMC7097526
- DOI: 10.1038/nri2054
Scientific barriers to developing vaccines against avian influenza viruses
Abstract
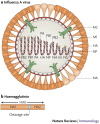
The increasing number of reports of direct transmission of avian influenza viruses to humans underscores the need for control strategies to prevent an influenza pandemic. Vaccination is the key strategy to prevent severe illness and death from pandemic influenza. Despite long-term experience with vaccines against human influenza viruses, researchers face several additional challenges in developing human vaccines against avian influenza viruses. In this Review, we discuss the features of avian influenza viruses, the gaps in our understanding of infections caused by these viruses in humans and of the immune response to them that distinguishes them from human influenza viruses, and the current status of vaccine development.
Conflict of interest statement
Kanta Subbarao and Tomy Joseph
Scientific barriers to developing vaccines against avian influenza viruses.
The laboratory of Dr Subbarao has a cooperative research and development agreement with MedImmune Vaccines to develop vaccines against potential pandemic strains of influenza.
Figures

References
-
- Wright PF, Webster RG. Fields Virology. 2001. pp. 1533–1579.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

