Mechanism of arachidonic acid action on syntaxin-Munc18
- PMID: 17363971
- PMCID: PMC1852766
- DOI: 10.1038/sj.embor.7400935
Mechanism of arachidonic acid action on syntaxin-Munc18
Abstract
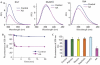
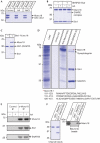
Syntaxin and Munc18 are, in tandem, essential for exocytosis in all eukaryotes. Recently, it was shown that Munc18 inhibition of neuronal syntaxin 1 can be overcome by arachidonic acid, indicating that this common second messenger acts to disrupt the syntaxin-Munc18 interaction. Here, we show that arachidonic acid can stimulate syntaxin 1 alone, indicating that it is syntaxin 1 that undergoes a structural change in the syntaxin 1-Munc18 complex. Arachidonic acid is incapable of dissociating Munc18 from syntaxin 1 and, crucially, Munc18 remains associated with syntaxin 1 after arachidonic-acid-induced syntaxin 1 binding to synaptosomal-associated protein 25 kDa (SNAP25). We also show that the same principle operates in the case of the ubiquitous syntaxin 3 isoform, highlighting the conserved nature of the mechanism of arachidonic acid action. Neuronal soluble N-ethyl maleimide sensitive factor attachment protein receptors (SNAREs) can be isolated from brain membranes in a complex with endogenous Munc18, consistent with a proposed function of Munc18 in vesicle docking and fusion.
Figures


References
-
- Brown WJ, Chambers K, Doody A (2003) Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic 4: 214–221 - PubMed
-
- Darios F, Davletov B (2006) ω-3 and ω-6 fatty acids stimulate cell membrane expansion by acting on syntaxin3. Nature 440: 813–817 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

