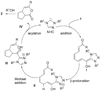
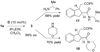
A highly enantioselective intramolecular Michael reaction catalyzed by N-heterocyclic carbenes
- PMID: 17366506
- PMCID: PMC2978499
- DOI: 10.1002/anie.200605235
A highly enantioselective intramolecular Michael reaction catalyzed by N-heterocyclic carbenes
Figures
Similar articles
-
Highly diastereo- and enantioselective additions of homoenolates to nitrones catalyzed by N-heterocyclic carbenes.J Am Chem Soc. 2008 Feb 27;130(8):2416-7. doi: 10.1021/ja710521m. Epub 2008 Jan 31. J Am Chem Soc. 2008. PMID: 18232690 Free PMC article. No abstract available.
-
Umpolung of Michael acceptors catalyzed by N-heterocyclic carbenes.J Am Chem Soc. 2006 Feb 8;128(5):1472-3. doi: 10.1021/ja058222q. J Am Chem Soc. 2006. PMID: 16448117 Free PMC article.
-
Organocatalysis by N-heterocyclic carbenes.Chem Rev. 2007 Dec;107(12):5606-55. doi: 10.1021/cr068372z. Epub 2007 Oct 23. Chem Rev. 2007. PMID: 17956132 Review. No abstract available.
-
Enantioselective Synthesis of Chromanones Bearing Quaternary Substituted Stereocenters Catalyzed by (1R)-Camphor-Derived N-Heterocyclic Carbenes.J Org Chem. 2015 Aug 7;80(15):7468-76. doi: 10.1021/acs.joc.5b01029. Epub 2015 Jul 21. J Org Chem. 2015. PMID: 26161638
-
Heterocyclic carbenes.Angew Chem Int Ed Engl. 2006 Feb 20;45(9):1348-52. doi: 10.1002/anie.200503858. Angew Chem Int Ed Engl. 2006. PMID: 16447297 Review. No abstract available.
Cited by
-
N-heterocyclic carbene-catalyzed oxidation of unactivated aldehydes to esters.Org Lett. 2008 Oct 2;10(19):4331-4. doi: 10.1021/ol8018488. Epub 2008 Aug 30. Org Lett. 2008. PMID: 18759433 Free PMC article.
-
NHC-catalyzed reactions of aryloxyacetaldehydes: a domino elimination/conjugate addition/acylation process for the synthesis of substituted coumarins.Org Lett. 2009 Jan 1;11(1):105-8. doi: 10.1021/ol802448c. Org Lett. 2009. PMID: 19049403 Free PMC article.
-
Catalytic dynamic kinetic resolutions with N-heterocyclic carbenes: asymmetric synthesis of highly substituted β-lactones.Angew Chem Int Ed Engl. 2012 Jul 16;51(29):7309-13. doi: 10.1002/anie.201203382. Epub 2012 Jun 14. Angew Chem Int Ed Engl. 2012. PMID: 22700327 Free PMC article.
-
Enantioselective synthesis of alpha,alpha-disubstituted cyclopentenes by an N-heterocyclic carbene-catalyzed desymmetrization of 1,3-diketones.J Am Chem Soc. 2007 Aug 22;129(33):10098-9. doi: 10.1021/ja073987e. Epub 2007 Jul 31. J Am Chem Soc. 2007. PMID: 17663558 Free PMC article. No abstract available.
-
Stereodivergency of triazolium and imidazolium-derived NHCs for catalytic, enantioselective cyclopentane synthesis.Org Lett. 2009 Feb 5;11(3):677-80. doi: 10.1021/ol802739d. Org Lett. 2009. PMID: 19175349 Free PMC article.
References
-
- Michael A. J Prakt. Chem. 1887;36:349–356.
- Bergmann ED, Ginsburg D, Pappo R. Org. React. 1959;10:179–555.
- Oare DA, Heathcock CA. In: Topics in Stereochemistry. Eliel EL, Wilen SH, editors. Vol. 20. New York: Wiley; 1991. pp. 124–170.
- Little RD, Masjedizadeh MR. Org. React. 1995;47:315–552.
- Krause N, Hoffmann-Röder A. Synthesis. 2001:171–196.
-
- Narasaka K, Soai K, Mukaiyama T. Chem. Lett. 1974:1223–1224.
- Mukaiyama T, Kobayashi S. Org. React. 1994;46:1–103.
- Evans DA, Scheidt KA, Johnston JN, Willis MC. J. Am. Chem. Soc. 2001;123:4480–4491. and references therein. - PubMed
- Jacobsen EN, Pfaltz A, Yamamoto H, editors. Comprehensive Asymmetric Catalysis. New York: Springer; 1999. chap. 31.
-
- Yamaguchi M, Shiraishi T, Hirama M. Angew. Chem. 1993;105:1243–1245. Angew. Chem. Int. Ed. Engl.1993, 32, 1176 – 1178.
- Yamaguchi M, Shiraishi T, Hirama M. J. Org. Chem. 1996;61:3520–3530.
-
- Brown SP, Goodwin NC, MacMillan DWC. J. Am. Chem. Soc. 2003;125:1192–1194. - PubMed
-
- Melchiorre P, Jørgensen KA. J. Org. Chem. 2003;68:4151–4157. - PubMed
- Halland N, Aburel PS, Jørgensen KA. Angew. Chem. 2003;115:685–689. Angew. Chem. Int. Ed.2003, 42, 661 – 665.
- Halland N, Aburel PS, Jørgensen KA. Angew. Chem. 2004;116:1292–1297. Angew. Chem. Int. Ed.2004, 43, 1272 – 1277.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources