Domain swapping reveals that the C- and N-terminal domains of DnaG and DnaB, respectively, are functional homologues
- PMID: 17367384
- PMCID: PMC3035176
- DOI: 10.1111/j.1365-2958.2007.05617.x
Domain swapping reveals that the C- and N-terminal domains of DnaG and DnaB, respectively, are functional homologues
Abstract
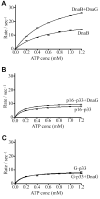
The bacterial primase (DnaG)-helicase (DnaB) interaction is mediated by the C-terminal domain of DnaG (p16) and a linker that joins the N- and C-terminal domains (p17 and p33 respectively) of DnaB. The crystal and nuclear magnetic resonance structures of p16 from Escherichia coli and Bacillus stearothermophilus DnaG proteins revealed a unique structural homology with p17, despite the lack of amino acid sequence similarity. The functional significance of this is not clear. Here, we have employed a 'domain swapping' approach to replace p17 with its structural homologue p16 to create chimeras. p33 alone hydrolyses ATP but exhibits no helicase activity. Fusing p16 (p16-p33) or DnaG (G-p33) to the N-terminus of p33 produced chimeras with partially restored helicase activities. Neither chimera interacted with DnaG. The p16-p33 chimera formed hexamers while G-p33 assembled into tetramers. Furthermore, G-p33 and DnaB formed mixed oligomers with ATPase activity better than that of the DnaB/DnaG complex and helicase activity better than the sum of the individual DnaB and G-p33 activities but worse than that of the DnaB/DnaG complex. Our combined data provide direct evidence that p16 and p17 are not only structural but also functional homologues, albeit their amino acid composition differences are likely to influence their precise roles.
Figures






References
-
- Bhattacharyya S, Griep MA. DnaB helicase affects the initiation specificity of E. coli primase on single-stranded DNA templates. Biochem. 2000;39:745–752. - PubMed
-
- Bird LE, Wigley DB. The B. stearothermophilus replicative helicase: cloning, overexpression and activity. Biochim Bioph Acta. 1999;1444:424–428. - PubMed
-
- Biswas SB, Chen PH, Biswas EE. Structure and function of E. coli DnaB protein: role of the N-terminal domain in helicase activity. Biochem. 1994;33:11307–11314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

