Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa
- PMID: 17367540
- PMCID: PMC1847430
- DOI: 10.1186/1471-2431-7-13
Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa
Abstract
Background: Few studies address the use of paediatric highly active antiretroviral therapy (HAART) in Africa.
Methods: We performed a retrospective cohort study to investigate preliminary outcomes of all children eligible for HAART at Sinikithemba HIV/AIDS clinic in KwaZulu-Natal, South Africa. Immunologic, virologic, clinical, mortality, primary caregiver, and psychosocial variables were collected and analyzed.
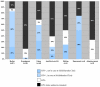
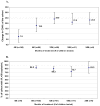
Results: From August 31, 2003 until October 31, 2005, 151 children initiated HAART. The median age at HAART initiation was 5.7 years (range 0.3-15.4). Median follow-up time of the cohort after HAART initiation was 8 months (IQR 3.5-13.5). The median change in CD4% from baseline (p < 0.001) was 10.2 (IQR 5.0-13.8) at 6 months (n = 90), and 16.2 (IQR 9.6-20.3) at 12 months (n = 59). Viral loads (VLs) were available for 100 children at 6 months of which 84% had HIV-1 RNA levels < or = 50 copies/mL. At 12 months, 80.3% (n = 61) had undetectable VLs. Sixty-five out of 88 children (73.8%) reported a significant increase (p < 0.001) in weight after the first month. Eighty-nine percent of the cohort (n = 132) reported < or = 2 missed doses during any given treatment month (> 95%adherence). Seventeen patients (11.3%) had a regimen change; two (1.3%) were due to antiretroviral toxicity. The Kaplan-Meier one year survival estimate was 90.9% (95%confidence interval (CI) 84.8-94.6). Thirteen children died during follow-up (8.6%), one changed service provider, and no children were lost to follow-up. All 13 deaths occurred in children with advanced HIV disease within 5 months of treatment initiation. In multivariate analysis of baseline variables against mortality using Cox proportional-hazards model, chronic gastroenteritis was associated with death [hazard ratio (HR), 12.34; 95% CI, 1.27-119.71) and an HIV-positive primary caregiver was found to be protective against mortality [HR, 0.12; 95% CI, 0.02-0.88). Age, orphanhood, baseline CD4%, and hemoglobin were not predicators of mortality in our cohort. Fifty-two percent of the cohort had at least one HIV-positive primary caregiver, and 38.4% had at least one primary caregiver also on HAART at Sinikithemba clinic.
Conclusion: This report suggests that paediatric HAART can be effective despite the challenges of a resource-limited setting.
Figures

Similar articles
-
Clinical outcome of HIV-infected patients with sustained virologic response to antiretroviral therapy: long-term follow-up of a multicenter cohort.PLoS One. 2006 Dec 20;1(1):e89. doi: 10.1371/journal.pone.0000089. PLoS One. 2006. PMID: 17183720 Free PMC article. Clinical Trial.
-
Two-year outcomes of children on non-nucleoside reverse transcriptase inhibitor and protease inhibitor regimens in a South African pediatric antiretroviral program.Pediatr Infect Dis J. 2008 Nov;27(11):993-8. doi: 10.1097/INF.0b013e31817acf7b. Pediatr Infect Dis J. 2008. PMID: 18818556
-
Long-term effectiveness of highly active antiretroviral therapy on the survival of children and adolescents with HIV infection: a 10-year follow-up study.Clin Infect Dis. 2008 Feb 15;46(4):507-15. doi: 10.1086/526524. Clin Infect Dis. 2008. PMID: 18199042
-
Lessons learned from use of highly active antiretroviral therapy in Africa.Clin Infect Dis. 2005 Aug 1;41(3):376-85. doi: 10.1086/431482. Epub 2005 Jun 30. Clin Infect Dis. 2005. PMID: 16007536 Review.
-
Very late initiation of HAART impairs treatment response at 48 and 96 weeks: results from a meta-analysis of randomized clinical trials.J Antimicrob Chemother. 2012 Feb;67(2):312-21. doi: 10.1093/jac/dkr478. Epub 2011 Nov 29. J Antimicrob Chemother. 2012. PMID: 22127587 Review.
Cited by
-
Variability of growth in children starting antiretroviral treatment in southern Africa.Pediatrics. 2012 Oct;130(4):e966-77. doi: 10.1542/peds.2011-3020. Epub 2012 Sep 17. Pediatrics. 2012. PMID: 22987878 Free PMC article.
-
Effect of Age at Antiretroviral Therapy Initiation on Catch-up Growth Within the First 24 Months Among HIV-infected Children in the IeDEA West African Pediatric Cohort.Pediatr Infect Dis J. 2015 Jul;34(7):e159-68. doi: 10.1097/INF.0000000000000734. Pediatr Infect Dis J. 2015. PMID: 25955835 Free PMC article.
-
Implementation of a comprehensive program including psycho-social and treatment literacy activities to improve adherence to HIV care and treatment for a pediatric population in Kenya.BMC Pediatr. 2008 Nov 21;8:52. doi: 10.1186/1471-2431-8-52. BMC Pediatr. 2008. PMID: 19025581 Free PMC article.
-
Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub-Saharan Africa: a narrative review.J Int AIDS Soc. 2015 Sep 16;18(1):20049. doi: 10.7448/IAS.18.1.20049. eCollection 2015. J Int AIDS Soc. 2015. PMID: 26385853 Free PMC article. Review.
-
Utility of total lymphocyte count as a surrogate marker for CD4 counts in HIV-1 infected children in Kenya.BMC Infect Dis. 2011 Sep 30;11:259. doi: 10.1186/1471-2334-11-259. BMC Infect Dis. 2011. PMID: 21961890 Free PMC article.
References
-
- AIDS epidemic update: December 2005. Geneva , United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization; 2005.
-
- Starr SE, Fletcher CV, Spector SA, Yong FH, Fenton T, Brundage RC, Manion D, Ruiz N, Gersten M, Becker M, McNamara J, Mofenson LM, Purdue L, Siminski S, Graham B, Kornhauser DM, Fiske W, Vincent C, Lischner HW, Dankner WM, Flynn PM. Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. Pediatric AIDS Clinical Trials Group 382 Team. N Engl J Med. 1999;341:1874–1881. doi: 10.1056/NEJM199912163412502. - DOI - PubMed
-
- van Rossum AM, Geelen SP, Hartwig NG, Wolfs TF, Weemaes CM, Scherpbier HJ, van Lochem EG, Hop WC, Schutten M, Osterhaus AD, Burger DM, de Groot R. Results of 2 years of treatment with protease-inhibitor--containing antiretroviral therapy in dutch children infected with human immunodeficiency virus type 1. Clin Infect Dis. 2002;34:1008–1016. doi: 10.1086/339443. - DOI - PubMed
-
- Resino S, Resino R, Maria Bellon J, Micheloud D, Gutierrez MD, de Jose MI, Ramos JT, Fontelos PM, Ciria L, Munoz-Fernandez MA. Clinical outcomes improve with highly active antiretroviral therapy in vertically HIV type-1-infected children. Clin Infect Dis. 2006;43:243–252. doi: 10.1086/505213. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

