Hes1 is required for pituitary growth and melanotrope specification
- PMID: 17367776
- PMCID: PMC1913046
- DOI: 10.1016/j.ydbio.2006.11.010
Hes1 is required for pituitary growth and melanotrope specification
Abstract
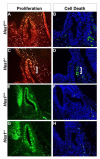
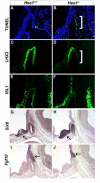
Rathke's pouch contains progenitor cells that differentiate into the endocrine cells of the pituitary gland. It gives rise to gonadotrope, thyrotrope, somatotrope, corticotrope and lactotrope cells in the anterior lobe and the intermediate lobe melanotropes. Pituitary precursor cells express many members of the Notch signaling pathway including the downstream effector gene Hes1. We hypothesized that Hes1 regulates the timing of precursor differentiation and cell fate determination. To test this idea, we expressed Hes1 in differentiating pituitary cells and found that it can inhibit gonadotrope and thyrotrope differentiation. Pituitaries of Hes1 deficient mice have anterior lobe hypoplasia. All cells in the anterior lobe are specified and differentiate, but an early period of increased cell death and reduced proliferation causes reduced growth, evident as early as e14.5. In addition, cells within the intermediate lobe differentiate into somatotropes instead of melanotropes. Thus, the Hes1 repressor is essential for melanotrope specification. These results demonstrate that Notch signaling plays multiple roles in pituitary development, influencing precursor number, organ size, cell differentiation and ultimately cell fate.
Figures






Similar articles
-
Hes1 and Hes5 are required for differentiation of pituicytes and formation of the neurohypophysis in pituitary development.Brain Res. 2015 Nov 2;1625:206-17. doi: 10.1016/j.brainres.2015.08.045. Epub 2015 Sep 6. Brain Res. 2015. PMID: 26348989
-
Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development.Mol Endocrinol. 2007 Jun;21(6):1458-66. doi: 10.1210/me.2007-0039. Epub 2007 Apr 10. Mol Endocrinol. 2007. PMID: 17426285
-
Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1.Dev Biol. 2009 Jan 1;325(1):151-61. doi: 10.1016/j.ydbio.2008.10.010. Epub 2008 Nov 1. Dev Biol. 2009. PMID: 18996108 Free PMC article.
-
Expression dynamics and functions of Hes factors in development and diseases.Curr Top Dev Biol. 2014;110:263-83. doi: 10.1016/B978-0-12-405943-6.00007-5. Curr Top Dev Biol. 2014. PMID: 25248479 Review.
-
The bHLH gene Hes1 regulates differentiation of multiple cell types.Mol Cells. 2000 Feb 29;10(1):1-7. doi: 10.1007/s10059-000-0001-0. Mol Cells. 2000. PMID: 10774739 Review.
Cited by
-
Notch-Dependent Pituitary SOX2(+) Stem Cells Exhibit a Timed Functional Extinction in Regulation of the Postnatal Gland.Stem Cell Reports. 2015 Dec 8;5(6):1196-1209. doi: 10.1016/j.stemcr.2015.11.001. Stem Cell Reports. 2015. PMID: 26651607 Free PMC article.
-
Persistent expression of activated notch in the developing hypothalamus affects survival of pituitary progenitors and alters pituitary structure.Dev Dyn. 2015 Aug;244(8):921-34. doi: 10.1002/dvdy.24283. Dev Dyn. 2015. PMID: 25907274 Free PMC article.
-
Analysis of differential gene expression in plurihormonal pituitary adenomas using bead-based fiber-optic arrays.J Neurooncol. 2012 Jul;108(3):341-8. doi: 10.1007/s11060-011-0792-1. Epub 2012 May 16. J Neurooncol. 2012. PMID: 22588334
-
Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice.Dev Biol. 2008 Jan 1;313(1):118-29. doi: 10.1016/j.ydbio.2007.10.006. Epub 2007 Oct 11. Dev Biol. 2008. PMID: 18037398 Free PMC article.
-
Genetic regulation of pituitary gland development in human and mouse.Endocr Rev. 2009 Dec;30(7):790-829. doi: 10.1210/er.2009-0008. Epub 2009 Oct 16. Endocr Rev. 2009. PMID: 19837867 Free PMC article. Review.
References
-
- Akazawa C, Sasai Y, Nakanishi S, Kageyama R. Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J Biol Chem. 1992;267:21879–85. - PubMed
-
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–81. - PubMed
-
- Bae S, Bessho Y, Hojo M, Kageyama R. The bHLH gene Hes6, an inhibitor of Hes1, promotes neuronal differentiation. Development. 2000;127:2933–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

