Cellular responses to EGFR inhibitors and their relevance to cancer therapy
- PMID: 17367921
- PMCID: PMC1986742
- DOI: 10.1016/j.canlet.2007.02.006
Cellular responses to EGFR inhibitors and their relevance to cancer therapy
Abstract
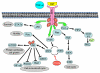
EGFR is a trans-membrane receptor tyrosine kinase that belongs to the HER family of receptors. The EGFR family plays an essential role in normal organ development by mediating morphogenesis and differentiation. Unlike normal cells that have tight regulatory mechanisms controlling EGFR pathways, tumor cells often have dysregulated EGFR signaling through receptor overexpression and/or mutation. This leads to proliferation under adverse conditions, invasion of surrounding tissues, and increased angiogenesis as well as resistance to radiation and chemotherapy. Therefore, EGFR is a legitimate therapeutic target. Numerous EGFR inhibitors are under development, but to date only four of them are FDA-approved, including two that inhibit the receptor's intracellular tyrosine kinase activity (gefitinib and erlotinib) and two that block extracellular ligand binding (cetuximab, and most recently panitumumab). In this review, we focus on how these different inhibitors affect EGFR signaling and the mechanisms by which they potentiate the effects of chemotherapy and radiation therapy. Numerous clinical trials have been conducted with these agents either as monotherapy, in combination with chemotherapy, or concurrently with radiation. Unfortunately, many of the clinical trials reported so far have shown at best limited gains; therefore, understanding the actions of these agents is essential to improving their efficacy in the treatment of cancers.
Figures
References
-
- Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist. 2002;7(Suppl 4):2–8. - PubMed
-
- Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102:37–46. - PubMed
-
- Yarden Y. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–8. - PubMed
-
- Riese DJ, Kim ED, Elenius K, Buckley S, Klagsbrun M, Plowman GD, et al. The epidermal growth factor receptor couples transforming growth factor-alpha, heparin-binding epidermal growth factor-like factor, and amphiregulin to neu, ErbB-3, and ErbB-4. J Biol Chem. 1996;271:20047–20052. - PubMed
-
- Riese DJ, II, Komurasaki T, Plowman GD, Stern DF. Activation of ErbB4 by the bifunctional epidermal growth factor family hormone epiregulin is regulated by ErbB2. J Biol Chem. 1998;273:11288–11294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

