Distribution of serotonin 5-HT2C receptors in the ventral tegmental area
- PMID: 17367945
- PMCID: PMC1939890
- DOI: 10.1016/j.neuroscience.2006.12.071
Distribution of serotonin 5-HT2C receptors in the ventral tegmental area
Abstract
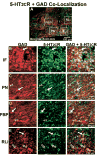
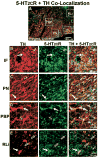
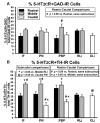
Serotonin 2C receptors (5-HT2CR) appear to exert tonic inhibitory influence over dopamine (DA) neurotransmission in the ventral tegmental area (VTA), the origin of the mesolimbic DA system, thought to be important in psychiatric disorders including addiction and schizophrenia. Current literature suggests that the inhibitory influence of 5-HT2CR on DA neurotransmission occurs via indirect activation of GABA inhibitory neurons, rather than via a direct action of 5-HT2CR on DA neurons. The present experiments were performed to establish the distribution of 5-HT2CR protein on DA and GABA neurons in the VTA of male rats via double-label immunofluorescence techniques. The 5-HT2CR protein was found to be co-localized with the GABA synthetic enzyme glutamic acid decarboxylase (GAD), confirming the presence of the 5-HT2CR on GABA neurons within the VTA. The 5-HT2CR immunoreactivity was also present in cells that contained immunoreactivity for tyrosine hydroxylase (TH), the DA synthetic enzyme, validating the localization of 5-HT2CR to DA neurons in the VTA. While the degree of 5-HT2CR+GAD co-localization was similar across the rostro-caudal levels of VTA subnuclei, 5-HT2CR+TH co-localization was highest in the middle relative to rostral and caudal levels of the VTA, particularly in the paranigral, parabrachial, and interfascicular subnuclei. The present results suggest that the inhibitory influence of the 5-HT2CR over DA neurotransmission in the VTA is a multifaceted and complex interplay of 5-HT2CR control of the output of both GABA and DA neurons within this region.
Figures


References
-
- Abramowski D, Rigo M, Duc D, Hoyer D, Staufenbiel M. Localization of the 5-hydroxytryptamine2C receptor protein in human and rat brain using specific antisera. Neuropharmacology. 1995;34:1635–1645. - PubMed
-
- Bankson MG, Yamamoto BK. Serotonin-GABA interactions modulate MDMA-induced mesolimbic dopamine release. J Neurochem. 2004;91:852–859. - PubMed
-
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–1985. - PubMed
-
- Bubar MJ, Seitz PK, Thomas ML, Cunningham KA. Validation of a selective serotonin 5-HT(2C) receptor antibody for utilization in fluorescence immunohistochemistry studies. Brain Res. 2005;1063:105–113. - PubMed
-
- Cai L, Friedman N, Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006;440:358–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

