The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats
- PMID: 17367952
- PMCID: PMC1940329
- DOI: 10.1016/j.neuroscience.2007.01.056
The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats
Abstract
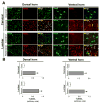
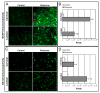
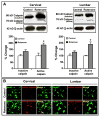
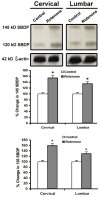
Exposure to environmental toxins increases the risk of neurodegenerative diseases including Parkinson's disease (PD). Rotenone is a neurotoxin that has been used to induce experimental Parkinsonism in rats. We used the rotenone model of experimental Parkinsonism to explore a novel aspect of extra-nigral degeneration, the neurodegeneration of spinal cord (SC), in PD. Rotenone administration to male Lewis rats caused significant neuronal cell death in cervical and lumbar SC as compared with control animals. Dying neurons were motoneurons as identified by double immunofluorescent labeling for terminal deoxynucleotidyl transferase, recombinant-mediated dUTP nick-end labeling-positive (TUNEL(+)) cells and choline acetyltransferase (ChAT)-immunoreactivity. Neuronal death was accompanied by abundant astrogliosis and microgliosis as evidenced from glial fibrillary acidic protein (GFAP)-immunoreactivity and OX-42-immunoreactivity, respectively, implicating an inflammatory component during neurodegeneration in SC. However, the integrity of the white matter in SC was not affected by rotenone administration as evidenced from the non co-localization of any TUNEL(+) cells with GFAP-immunoreactivity and myelin basic protein (MBP)-immunoreactivity, the selective markers for astrocytes and oligodendrocytes, respectively. Increased activities of 76 kD active m-calpain and 17/19 kD active caspase-3 further demonstrated involvement of these enzymes in cell death in SC. The finding of ChAT(+) cell death also suggested degeneration of SC motoneurons in rotenone-induced experimental Parkinsonism. Thus, this is the first report of its kind in which the selective vulnerability of a putative parkinsonian target outside of nigrostriatal system has been tested using an environmental toxin to understand the pathophysiology of PD. Moreover, rotenone-induced degeneration of SC motoneuron in this model of experimental Parkinsonism progressed with upregulation of calpain and caspase-3.
Figures






Similar articles
-
Immunofluorescent labeling of increased calpain expression and neuronal death in the spinal cord of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice.Brain Res. 2004 May 1;1006(2):150-6. doi: 10.1016/j.brainres.2004.01.065. Brain Res. 2004. PMID: 15051518
-
Calpain inhibition protected spinal cord motoneurons against 1-methyl-4-phenylpyridinium ion and rotenone.Neuroscience. 2011 Sep 29;192:263-74. doi: 10.1016/j.neuroscience.2011.06.007. Epub 2011 Jun 22. Neuroscience. 2011. PMID: 21723922 Free PMC article.
-
Calpain activation in apoptosis of motoneurons in cell culture models of experimental parkinsonism.Ann N Y Acad Sci. 2006 Aug;1074:349-56. doi: 10.1196/annals.1369.034. Ann N Y Acad Sci. 2006. PMID: 17105932
-
Calpain activation and progression of inflammatory cycles in Parkinson's disease.Front Biosci (Landmark Ed). 2022 Jan 13;27(1):20. doi: 10.31083/j.fbl2701020. Front Biosci (Landmark Ed). 2022. PMID: 35090325 Free PMC article. Review.
-
Calpain as a potential therapeutic target in Parkinson's disease.CNS Neurol Disord Drug Targets. 2008 Jun;7(3):305-12. doi: 10.2174/187152708784936680. CNS Neurol Disord Drug Targets. 2008. PMID: 18673214 Review.
Cited by
-
Should we reconsider the apoptosis as a strategic player in tissue regeneration?Int J Biol Sci. 2019 Jul 25;15(10):2029-2036. doi: 10.7150/ijbs.36362. eCollection 2019. Int J Biol Sci. 2019. PMID: 31592227 Free PMC article. Review.
-
Inhibitory effect of PACAP on caspase activity in neuronal apoptosis: a better understanding towards therapeutic applications in neurodegenerative diseases.J Mol Neurosci. 2008 Nov;36(1-3):26-37. doi: 10.1007/s12031-008-9087-1. Epub 2008 May 28. J Mol Neurosci. 2008. PMID: 18506634 Review.
-
Rosinidin inhibits NF-κB/ Nrf2/caspase-3 expression and restores neurotransmitter levels in rotenone-activated Parkinson's disease.Saudi J Biol Sci. 2023 Jun;30(6):103656. doi: 10.1016/j.sjbs.2023.103656. Epub 2023 Apr 23. Saudi J Biol Sci. 2023. PMID: 37187936 Free PMC article.
-
Melatonin attenuates calpain upregulation, axonal damage and neuronal death in spinal cord injury in rats.J Pineal Res. 2008 May;44(4):348-57. doi: 10.1111/j.1600-079X.2007.00534.x. Epub 2007 Dec 13. J Pineal Res. 2008. PMID: 18086148 Free PMC article.
-
Inhibition of Apoptosis and Efficacy of Pan Caspase Inhibitor, Q-VD-OPh, in Models of Human Disease.J Cell Death. 2015 Apr 8;8:1-7. doi: 10.4137/JCD.S23844. eCollection 2015. J Cell Death. 2015. PMID: 25922583 Free PMC article. Review.
References
-
- Ahmadi FA, Linseman DA, Grammatopoulos TN, Jones SM, Bouchard RJ, Freed CR, Heidenreich KA, Zawada WM. The pesticide rotenone induces caspase-3-mediated apoptosis in ventral mesencephalic dopaminergic neurons. J Neurochem. 2003;87:914–921. - PubMed
-
- Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res. 2002;136:317–324. - PubMed
-
- Andreassen OA, Ferrante RJ, Klivenyi P, Klein AM, Dedeoglu A, Albers DS, Kowall NW, Beal MF. Transgenic ALS mice show increased vulnerability to the mitochondrial toxins MPTP and 3-nitropropionic acid. Exp Neurol. 2001;168:356–363. - PubMed
-
- Banik NL, Hogan EL, Jenkins MG, McDonald JK, McAlhaney WW, Sostek MB. Purification of a calcium-activated neutral proteinase from bovine brain. Neurochem Res. 1983;8:1389–1405. - PubMed
-
- Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:120–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

