The microtubule-associated protein doublecortin is broadly expressed in the telencephalon of adult canaries
- PMID: 17367992
- PMCID: PMC2040224
- DOI: 10.1016/j.jchemneu.2007.02.002
The microtubule-associated protein doublecortin is broadly expressed in the telencephalon of adult canaries
Abstract
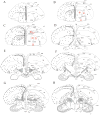
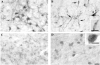
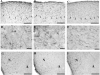
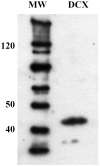
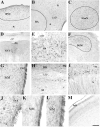
The protein doublecortin (DCX) is expressed in post-mitotic migrating and differentiating neurons in the developing vertebrate brain and, as a part of the microtubule machinery, is required for neuronal migration. DCX expression is generally maximal during embryonic and early post-natal life but decreases markedly and almost disappears in older animals in parallel with the major decrease or cessation of neurogenesis. In several seasonally breeding songbird species such as canaries, the volume of several song control nuclei in the brain varies seasonally such that the largest nuclei are observed in the late spring and early summer. This variation is based on changes in cell size, dendritic branching, and, in nucleus HVC, on the incorporation of neurons newly born in adulthood. Because songbirds maintain an active neurogenesis and neuronal incorporation in their telencephalon throughout their lives, we investigated here the distribution of DCX-immunoreactive (ir) structures in the brain of adult male canaries. Densely stained DCX-ir cells were found exclusively in parts of the telencephalon that are known to incorporate new neurons in adulthood, in particular the nidopallium. Within this brain region, the boundaries of the song control nucleus HVC could be clearly distinguished from surrounding structures by a higher density of DCX-ir structures. In most telencephalic areas, about two thirds of these cells displayed a uni- or bipolar fusiform morphology suggesting they were migrating neurons. The rest of the DCX-ir cells in the telencephalon were larger and had a round multipolar morphology. No such staining was found in the rest of the brain. The broad expression of DCX specifically in adult brain structures that exhibit the characteristic of active incorporation of new neurons suggests that DCX plays a key role in the migration of new neurons in the brain of adult songbirds as it presumably does during ontogeny.
Figures


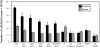
References
-
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990;249:1444–1446. - PubMed
-
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–83. - PubMed
-
- Ball GF. Neuroendocrine basis of seasonal changes in vocal behavior among songbirds. In: Hauser M, Konishi M, editors. The design of animal communication. MIT Press; Cambridge,MA: 1999. pp. 213–253.
-
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal Plasticity in the song control system. Multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann NY Acad Sci. 2004;1016:1–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

