Molecular imaging research in the outcomes era: measuring outcomes for individualized cancer therapy
- PMID: 17368207
- PMCID: PMC1868571
- DOI: 10.1016/j.acra.2007.01.005
Molecular imaging research in the outcomes era: measuring outcomes for individualized cancer therapy
Abstract
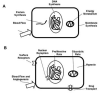
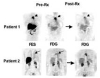
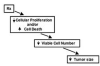
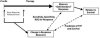
Advances in molecular imaging, combined with the goal of personalized cancer therapy, call for new approaches to clinical study design for trials testing imaging to guide therapy. The role of cancer imaging must expand and move beyond tumor detection and localization to incorporate quantitative evaluation of regional tumor phenotype. Imaging study design and outcome analysis must move beyond metrics designed to measure the performance for detection to include measures of prognosis, prediction of therapeutic success, and early therapy response. This implies changes in how studies are carried and out, and importantly in the regulatory oversight of cancer imaging. Demonstration that a biochemical or molecular imaging method correctly and accurately measures a specific biologic feature should be sufficient for approval for clinical trials. It may be possible that a combination of imaging procedures known to accurately depict tumor phenotype may be prognostic, even if the individual study cannot be directly validated against patient outcomes. Therefore, it will be important to be able to apply a range of possible imaging studies to different targeted cancer therapy trials. Academia and industry must work together with regulatory agencies and payers to facilitate well designed clinical studies, with appropriate outcome measures, to test the effectiveness of imaging in helping to direct cancer therapy. These will assure the appropriate use of imaging to direct treatment and make an important step towards individualized cancer therapy.
Figures


References
-
- Kaklamani V, O′Regan RM. New targeted therapies in breast cancer. Semin Oncol. 2004;31(2 Suppl 4):20–5. - PubMed
-
- Sawyers CL. Making progress through molecular attacks on cancer. Cold Spring Harb Symp Quant Biol. 2005;70:479–82. - PubMed
-
- Beckman RA, Loeb LA. Genetic instability in cancer: theory and experiment. Semin Cancer Biol. 2005;15(6):423–35. - PubMed
-
- Aboagye EO, Price PM. Use of positron emission tomography in anticancer drug development. Invest New Drugs. 2003;21(2):169–81. - PubMed
-
- Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O′Shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11(8):2785–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

