Laminar shear stress up-regulates the expression of stearoyl-CoA desaturase-1 in vascular endothelial cells
- PMID: 17368438
- PMCID: PMC2791953
- DOI: 10.1016/j.cardiores.2007.02.014
Laminar shear stress up-regulates the expression of stearoyl-CoA desaturase-1 in vascular endothelial cells
Abstract
Objective: Laminar shear stress plays critical roles in vascular homeostasis and exerts various metabolic effects on endothelial cells (ECs). Stearoyl-CoA desaturase-1 (SCD1), which catalyzes the biosynthesis of monounsaturated fatty acids, affects the lipid composition and fluidity of the cell membrane. Thus, we examined the effect of laminar flow on SCD1 expression in ECs.
Methods: A flow chamber was used to impose a laminar shear stress on a confluent monolayer of human vascular ECs. The expression of SCD1 was examined using real-time RT-PCR and Northern and Western blotting. Immunohistochemical staining was used to assess the expression of SCD1 in Sprague-Dawley rat arteries, including the sites of arterial bifurcation.
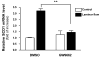
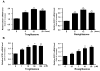
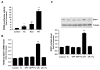
Results: Laminar shear stress (12 dyn/cm2, 12 h) markedly increased the gene expression of SCD1 in ECs. The flow-induced SCD1 expression was attenuated by peroxisome proliferator-activated receptor (PPAR)-gamma antagonists both in vitro and in vivo. Troglitazone and rosiglitazone significantly increased the gene expression of SCD1. Furthermore, overexpression of a constitutively active PPARgamma induced the expression of SCD1 in ECs. Immunohistochemical study of cross-sections from rat celiac arteries revealed that endothelial expression of SCD1 was substantially higher on the medial division apex, where the shear stress is high and more laminar, than the lateral aspect, where the shear stress is low and unsteady.
Conclusion: These in vitro and in vivo results demonstrate that laminar flow increased the expression of SCD1 in endothelium through a PPARgamma-specific mechanism, which may contribute to the shear stress-mediated protective roles in ECs.
Figures
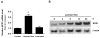

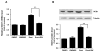


References
-
- Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–9. - PubMed
-
- Haidekker MA, L’Heureux N, Frangos JA. Fluid shear stress increases membrane fluidity in endothelial cells: a study with DCVJ fluorescence. Am J Physiol Heart Circ Physiol. 2000;278:H1401–6. - PubMed
-
- Butler PJ, Norwich G, Weinbaum S, Chien S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am J Physiol Cell Physiol. 2001;280:C962–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

