Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury
- PMID: 17368446
- PMCID: PMC1995439
- DOI: 10.1016/j.expneurol.2007.01.035
Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury
Abstract
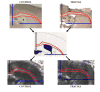
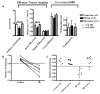
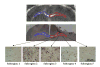
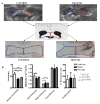
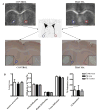
Traumatic axonal injury (TAI) is thought to be a major contributor to cognitive dysfunction following traumatic brain injury (TBI), however TAI is difficult to diagnose or characterize non-invasively. Diffusion tensor imaging (DTI) has shown promise in detecting TAI, but direct comparison to histologically-confirmed axonal injury has not been performed. In the current study, mice were imaged with DTI, subjected to a moderate cortical controlled impact injury, and re-imaged 4-6 h and 24 h post-injury. Axonal injury was detected by amyloid beta precursor protein (APP) and neurofilament immunohistochemistry in pericontusional white matter tracts. The severity of axonal injury was quantified using stereological methods from APP stained histological sections. Two DTI parameters--axial diffusivity and relative anisotropy--were significantly reduced in the injured, pericontusional corpus callosum and external capsule, while no significant changes were seen with conventional MRI in these regions. The contusion was easily detectable on all MRI sequences. Significant correlations were found between changes in relative anisotropy and the density of APP stained axons across mice and across subregions spanning the spatial gradient of injury. The predictive value of DTI was tested using a region with DTI changes (hippocampal commissure) and a region without DTI changes (anterior commissure). Consistent with DTI predictions, there was histological detection of axonal injury in the hippocampal commissure and none in the anterior commissure. These results demonstrate that DTI is able to detect axonal injury, and support the hypothesis that DTI may be more sensitive than conventional imaging methods for this purpose.
Figures




References
-
- Adams JH. Diffuse axonal injury in non-missile head injury. Injury. 1982;13:444–445. - PubMed
-
- Adams JH, Doyle D, Graham DI, Lawrence AE, McLellan DR. Diffuse axonal injury in head injuries caused by a fall. Lancet. 1984;2:1420–1422. - PubMed
-
- Adelson PD, Ragheb J, Kanev P, Brockmeyer D, Beers SR, Brown SD, Cassidy LD, Chang Y, Levin H. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740–754. discussion 740-754. - PubMed
-
- Bammer R, Augustin M, Strasser-Fuchs S, Seifert T, Kapeller P, Stollberger R, Ebner F, Hartung HP, Fazekas F. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magn Reson Med. 2000;44:583–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

