In vivo cellular repopulation of tubular elastin scaffolds mediated by basic fibroblast growth factor
- PMID: 17368531
- PMCID: PMC2262161
- DOI: 10.1016/j.biomaterials.2007.02.031
In vivo cellular repopulation of tubular elastin scaffolds mediated by basic fibroblast growth factor
Abstract
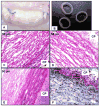
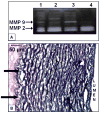
In vivo tissue engineering has been explored as a method to repopulate scaffolds with autologous cells to create a functional, living, and non-immunogenic tissue substitute. In this study, we describe an approach to in vivo cellular repopulation of a tissue-derived tubular elastin scaffold. Pure elastin scaffolds were prepared from porcine carotid arteries (elastin tubes). Elastin tubes were filled with agarose gel containing basic fibroblast growth factor (bFGF) to allow sustained release of growth factor. These tubes were implanted in subdermal pouches in adult rats. The elastin tubes with growth factor had significantly more cell infiltration at 28 days than those without growth factor. Immunohistochemical staining indicated that most of these cells were fibroblasts, of which a few were activated fibroblasts (myofibroblasts). Microvasculature was also observed within the scaffolds. Macrophage infiltration was seen at 7 days, which diminished by 28 days of implantation. None of the elastin tubes with bFGF calcified. These results demonstrated that the sustained release of bFGF brings about repopulation of elastin scaffolds in vivo while inhibiting calcification. Results showing myofibroblast infiltration and vascularization are encouraging since such an in vivo implantation technique could be used for autologous cell repopulation of elastin scaffolds for vascular graft applications.
Figures




References
-
- Association AH. Biostatistical factsheet: cardiovascular procedures. American Heart Association; 2003.
-
- Berglund JD, Mohseni MM, Nerem RM, Sambanis A. A biological hybrid model for collagen-based tissue engineered vascular constructs. Biomaterials. 2003 Mar;24(7):1241–54. - PubMed
-
- Seliktar D, et al. Dynamic mechanical conditioning of collagen gel blood vessel constructs induces remodeling in vitro. Ann Biomed Eng. 2000;28(4):351–62. - PubMed
-
- Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215–31. - PubMed
-
- Schaner P, Martin N, Tulenko T, Shapiro I, Tarola N, Leichter R, et al. Decellularized vein as a potential scaffold for vascular tissue engineering. Journal of Vascular Surgery. 2004;40( 1):146–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

