Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice
- PMID: 17368609
- PMCID: PMC1899534
- DOI: 10.1016/j.ydbio.2007.02.019
Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice
Abstract
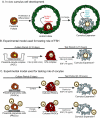
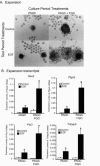
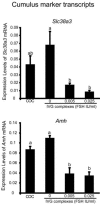
Preantral granulosa cells (PAGCs) differentiate into cumulus cells following antrum formation. Cumulus cells, but not PAGCs, are competent to undergo expansion. Experiments reported here tested the respective roles of both oocytes and FSH in the transition of preantral granulosa cells to cumulus cells competent to undergo expansion. PAGC-oocyte complexes were cultured with or without a low dose of FSH (0.005 IU/ml) and isolated PAGCs were cultured with or without oocytes. At the end of culture, complexes or isolated PAGCs were tested for their ability to undergo cumulus expansion and upregulate expansion transcripts in response to EGF or FSH (0.5 IU/ml). The ability to undergo expansion in response to EGF required the presence of oocytes but not FSH during the culture period. Likewise, complexes isolated from the ovaries of hypogonadal mice, which lack circulating gonadotropins, underwent expansion in response to EGF, but not FSH. In contrast, the ability to activate MAPK3/1 and MAPK14 and undergo expansion in response to FSH required prior exposure to low doses of FSH. However, these low levels (0.005 or 0.025 IU FSH/ml) suppressed expression of Slc38a3 and Amh, two transcripts highly expressed in cumulus cells, suggesting opposing effects of FSH on cumulus cell differentiation. In conclusion, the ability to undergo expansion in response to FSH requires prior exposure to FSH during development, while oocyte-derived factors alone are sufficient to promote the ability to undergo expansion in response to EGF. These results highlight the crucial role of oocytes in driving the differentiation of PAGCs into cumulus cells during the preantral to antral follicle transition.
Figures






Similar articles
-
The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion.Dev Biol. 2006 Nov 1;299(1):91-104. doi: 10.1016/j.ydbio.2006.07.012. Epub 2006 Jul 15. Dev Biol. 2006. PMID: 16908014
-
Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation.Dev Biol. 1990 Aug;140(2):307-17. doi: 10.1016/0012-1606(90)90081-s. Dev Biol. 1990. PMID: 2115479
-
FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte.Dev Biol. 1990 Mar;138(1):16-25. doi: 10.1016/0012-1606(90)90172-f. Dev Biol. 1990. PMID: 2155145
-
The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process.J Reprod Dev. 2012;58(5):510-4. doi: 10.1262/jrd.2012-056. J Reprod Dev. 2012. PMID: 23124701 Review.
-
Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism.Semin Reprod Med. 2009 Jan;27(1):32-42. doi: 10.1055/s-0028-1108008. Epub 2009 Feb 5. Semin Reprod Med. 2009. PMID: 19197803 Free PMC article. Review.
Cited by
-
The nuclear receptor cofactor receptor-interacting protein 140 is a positive regulator of amphiregulin expression and cumulus cell-oocyte complex expansion in the mouse ovary.Endocrinology. 2010 Jun;151(6):2923-32. doi: 10.1210/en.2010-0081. Epub 2010 Mar 22. Endocrinology. 2010. PMID: 20308529 Free PMC article.
-
The Function of Cumulus Cells in Oocyte Growth and Maturation and in Subsequent Ovulation and Fertilization.Cells. 2021 Sep 2;10(9):2292. doi: 10.3390/cells10092292. Cells. 2021. PMID: 34571941 Free PMC article. Review.
-
Quantitative morphokinetic parameters identify novel dynamics of oocyte meiotic maturation and cumulus expansion†.Biol Reprod. 2022 Oct 11;107(4):1097-1112. doi: 10.1093/biolre/ioac139. Biol Reprod. 2022. PMID: 35810327 Free PMC article.
-
Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions.Reprod Biol Endocrinol. 2010 Oct 14;8:119. doi: 10.1186/1477-7827-8-119. Reprod Biol Endocrinol. 2010. PMID: 20946661 Free PMC article. Review.
-
Activin promotes growth and antral cavity expansion in the dog ovarian follicle.Theriogenology. 2019 Apr 15;129:168-177. doi: 10.1016/j.theriogenology.2019.02.018. Epub 2019 Feb 25. Theriogenology. 2019. PMID: 30856402 Free PMC article.
References
-
- Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev. Biol. 1990;138:16–25. - PubMed
-
- Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol. Reprod. Dev. 1993;34:87–93. - PubMed
-
- Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: Development of competence to undergo expansion. Dev. Biol. 2006;299:91–104. - PubMed
-
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, SassoneCorsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. USA. 1998;95:13612–13617. - PMC - PubMed
-
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, Gilchrist RB. Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol. Reprod. 2006 DOI:10.1095/biolreprod.106.057471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

