Exchange of DNA polymerases at the replication fork of bacteriophage T7
- PMID: 17369350
- PMCID: PMC1838503
- DOI: 10.1073/pnas.0701062104
Exchange of DNA polymerases at the replication fork of bacteriophage T7
Abstract
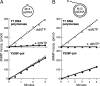
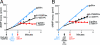
T7 gene 5 DNA polymerase (gp5) and its processivity factor, Escherichia coli thioredoxin, together with the T7 gene 4 DNA helicase, catalyze strand displacement synthesis on duplex DNA processively (>17,000 nucleotides per binding event). The processive DNA synthesis is resistant to the addition of a DNA trap. However, when the polymerase-thioredoxin complex actively synthesizing DNA is challenged with excess DNA polymerase-thioredoxin exchange occurs readily. The exchange can be monitored by the use of a genetically altered T7 DNA polymerase (gp5-Y526F) in which tyrosine-526 is replaced with phenylalanine. DNA synthesis catalyzed by gp5-Y526F is resistant to inhibition by chain-terminating dideoxynucleotides because gp5-Y526F is deficient in the incorporation of these analogs relative to the wild-type enzyme. The exchange also occurs during coordinated DNA synthesis in which leading- and lagging-strand synthesis occur at the same rate. On ssDNA templates with the T7 DNA polymerase alone, such exchange is not evident, suggesting that free polymerase is first recruited to the replisome by means of T7 gene 4 helicase. The ability to exchange DNA polymerases within the replisome without affecting processivity provides advantages for fidelity as well as the cycling of the polymerase from a completed Okazaki fragment to a new primer on the lagging strand.
Conflict of interest statement
Conflict of interest: C.C.R. is a consultant to General Electronics Corp., which has a license from Harvard University to commercialize DNA polymerase for DNA sequencing.
Figures

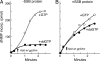
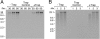

References
-
- Richardson CC. Cell. 1983;33:315–317. - PubMed
-
- Tabor S, Huber HE, Richardson CC. J Biol Chem. 1987;262:16212–16223. - PubMed
-
- Huber HE, Tabor S, Richardson CC. J Biol Chem. 1987;262:16224–16232. - PubMed
-
- Matson SW, Tabor S, Richardson CC. J Biol Chem. 1983;258:14017–14024. - PubMed
-
- Lechner RL, Richardson CC. J Biol Chem. 1983;258:11185–11196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

