Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint
- PMID: 17369399
- PMCID: PMC1820940
- DOI: 10.1101/gad.1511107
Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint
Abstract
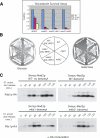
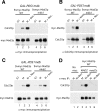
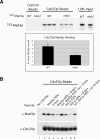
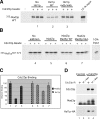
Inappropriate attachment/tension between chromosomal kinetochores and the kinetochore microtubules activates the spindle assembly checkpoint, which delays anaphase by blocking the ubiquitin-mediated degradation of securin/Pds1p by APCCdc20. The checkpoint proteins Mad2 and Mad3/BubR1 bind to Cdc20, although how they inhibit APCCdc20 is unclear. We investigated the roles of two evolutionarily conserved KEN boxes and a D box within Mad3/BubR1. Although such motifs usually mediate APC-substrate recognition and ubiquitination, they have no apparent role in Mad3p turnover in Saccharomyces cerevisiae. Instead, these motifs are important for Mad3p function in the checkpoint and for binding to Cdc20p. We show that the Mad3p D box and KEN boxes function together to mediate Cdc20p-Mad3p interaction and that Mad3p and an anaphase-promoting complex (APC) substrate, Hsl1p, compete for Cdc20p binding in a D-box- and KEN-box-dependent manner. In vivo, we observed an increased binding of Cdc20p to Mad3p and decreased binding to Hsl1p upon checkpoint activation. Furthermore, we demonstrate that Mad2p stimulates the association between Mad3p and Cdc20p and that this stimulated binding requires KEN box 1 within Mad3p. These findings implicate Mad3p as a pseudosubstrate inhibitor of APCCdc20, competing with APC substrates for Cdc20p binding. We present a model aimed at unifying previous analyses of checkpoint function by focusing on the Mad3-Cdc20 interaction.
Figures



References
-
- Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K., Moore D.D., Seidman J.G., Smith J.A., Struhl K., Seidman J.G., Smith J.A., Struhl K., Smith J.A., Struhl K., Struhl K. Current protocols in molecular biology. Wiley; New York: 1995.
-
- Burton J.L., Tsakraklides V., Solomon M.J., Tsakraklides V., Solomon M.J., Solomon M.J. Assembly of an APC–Cdh1–substrate complex is stimulated by engagement of a destruction box. Mol. Cell. 2005;18:533–542. - PubMed
-
- Carroll C.W., Enquist-Newman M., Morgan D.O., Enquist-Newman M., Morgan D.O., Morgan D.O. The APC subunit Doc1 promotes recognition of the substrate destruction box. Curr. Biol. 2005;15:11–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
