Miscibility and phase behavior of N-acylethanolamine/diacylphosphatidylethanolamine binary mixtures of matched acyl chainlengths (N=14, 16)
- PMID: 17369415
- PMCID: PMC1868988
- DOI: 10.1529/biophysj.106.096610
Miscibility and phase behavior of N-acylethanolamine/diacylphosphatidylethanolamine binary mixtures of matched acyl chainlengths (N=14, 16)
Abstract
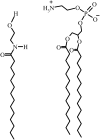
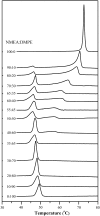
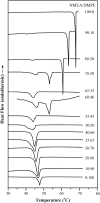
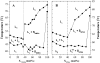
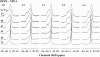
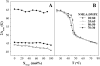
The miscibility and phase behavior of hydrated binary mixtures of two N-acylethanolamines (NAEs), N-myristoylethanolamine (NMEA), and N-palmitoylethanolamine (NPEA), with the corresponding diacyl phosphatidylethanolamines (PEs), dimyristoylphosphatidylethanolamine (DMPE), and dipalmitoylphosphatidylethanolamine (DPPE), respectively, have been investigated by differential scanning calorimetry (DSC), spin-label electron spin resonance (ESR), and (31)P-NMR spectroscopy. Temperature-composition phase diagrams for both NMEA/DMPE and NPEA/DPPE binary systems were established from high sensitivity DSC. The structures of the phases involved were determined by (31)P-NMR spectroscopy. For both systems, complete miscibility in the fluid and gel phases is indicated by DSC and ESR, up to 35 mol % of NMEA in DMPE and 40 mol % of NPEA in DPPE. At higher contents of the NAEs, extensive solid-fluid phase separation and solid-solid immiscibility occur depending on the temperature. Characterization of the structures of the mixtures formed with (31)P-NMR spectroscopy shows that up to 75 mol % of NAE, both DMPE and DPPE form lamellar structures in the gel phase as well as up to at least 65 degrees C in the fluid phase. ESR spectra of phosphatidylcholine spin labeled at the C-5 position in the sn-2 acyl chain present at a probe concentration of 1 mol % exhibit strong spin-spin broadening in the low-temperature region for both systems, suggesting that the acyl chains pack very tightly and exclude the spin label. However, spectra recorded in the fluid phase do not exhibit any spin-spin broadening and indicate complete miscibility of the two components. The miscibility of NAE and diacyl PE of matched chainlengths is significantly less than that found earlier for NPEA and dipalmitoylphosphatidylcholine, an observation that is consistent with the notion that the NAEs are most likely stored as their precursor lipids (N-acyl PEs) and are generated only when the system is subjected to membrane stress.
Figures
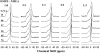
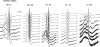
References
-
- Schmid, H. H. O., P. C. Schmid, and V. Natarajan. 1990. N-Acylated glycerophospholipids and their derivatives. Prog. Lipid Res. 29:1–43. - PubMed
-
- Hansen, H. S., B. Moesgaard, H. H. Hansen, and G. Petersen. 2000. N-Acylethanolamines and precursor phospholipids: relation to cell injury. Chem. Phys. Lipids. 108:135–150. - PubMed
-
- Schmid, H. H. O., P. C. Schmid, and V. Natarajan. 1996. The N-acylation-phosphodiesterase pathway and signaling. Chem. Phys. Lipids. 80:133–142. - PubMed
-
- Hansen, H. H., C. Ikonomidou, P. Bittigau, and H. S. Hansen. 2001. Accumulation of the anadamide precursor and other N-acylethanolamine phospholipids in infant rat models of in vivo necrotic and apoptotic neural death. J. Neurochem. 76:39–46. - PubMed
-
- Hansen, H. H., P. C. Schmid, P. Bittigau, I. Lastres-Becker, F. Berrendero, J. Manzanares, C. Ikonomidou, H. H. O. Schmid, J. J. Fernandez-Ruiz, and H. S. Hansen. 2001. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J. Neurochem. 78:1415–1427. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

