A molecular model for intercellular synchronization in the mammalian circadian clock
- PMID: 17369417
- PMCID: PMC1868999
- DOI: 10.1529/biophysj.106.094086
A molecular model for intercellular synchronization in the mammalian circadian clock
Abstract
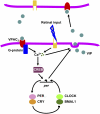
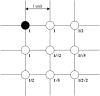
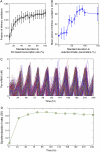
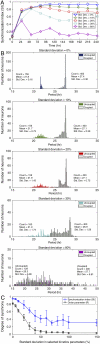
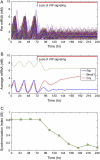
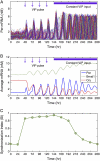
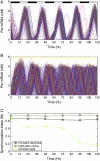
The mechanisms and consequences of synchrony among heterogeneous oscillators are poorly understood in biological systems. We present a multicellular, molecular model of the mammalian circadian clock that incorporates recent data implicating the neurotransmitter vasoactive intestinal polypeptide (VIP) as the key synchronizing agent. The model postulates that synchrony arises among circadian neurons because they release VIP rhythmically on a daily basis and in response to ambient light. Two basic cell types, intrinsically rhythmic pacemakers and damped oscillators, are assumed to arise from a distribution of Period gene transcription rates. Postsynaptic neurons show time-of-day dependent responses to VIP binding through a signaling cascade that activates Period mRNA transcription. The heterogeneous cell ensemble model self-synchronizes, entrains to ambient light-dark cycles, and desynchronizes in constant bright light or upon removal of VIP signaling. The degree of synchronicity observed depends on cell-specific features (e.g., mean and variability of parameters within the rhythm-generating loop), in addition to the more commonly studied effect of intercellular coupling strength. These simulations closely replicate experimental data and predict that heterogeneous oscillations (e.g., sustained, damped, and arrhythmic) arise from small differences in the molecular parameters between cells, that damped oscillators participate in entrainment and synchrony of the ensemble of cells, and that constant light desynchronizes oscillators by maximizing VIP release.
Figures
Similar articles
-
Small-world network models of intercellular coupling predict enhanced synchronization in the suprachiasmatic nucleus.J Biol Rhythms. 2009 Jun;24(3):243-54. doi: 10.1177/0748730409333220. J Biol Rhythms. 2009. PMID: 19465701 Free PMC article.
-
A neuropeptide speeds circadian entrainment by reducing intercellular synchrony.Proc Natl Acad Sci U S A. 2013 Nov 12;110(46):E4355-61. doi: 10.1073/pnas.1307088110. Epub 2013 Oct 28. Proc Natl Acad Sci U S A. 2013. PMID: 24167276 Free PMC article.
-
Multicellular model for intercellular synchronization in circadian neural networks.Biophys J. 2011 Jul 6;101(1):12-20. doi: 10.1016/j.bpj.2011.04.051. Biophys J. 2011. PMID: 21723810 Free PMC article.
-
Circadian organization of the mammalian retina: from gene regulation to physiology and diseases.Prog Retin Eye Res. 2014 Mar;39:58-76. doi: 10.1016/j.preteyeres.2013.12.001. Epub 2013 Dec 12. Prog Retin Eye Res. 2014. PMID: 24333669 Free PMC article. Review.
-
Mammalian circadian signaling networks and therapeutic targets.Nat Chem Biol. 2007 Oct;3(10):630-9. doi: 10.1038/nchembio.2007.37. Nat Chem Biol. 2007. PMID: 17876320 Review.
Cited by
-
A multiscale model to investigate circadian rhythmicity of pacemaker neurons in the suprachiasmatic nucleus.PLoS Comput Biol. 2010 Mar 12;6(3):e1000706. doi: 10.1371/journal.pcbi.1000706. PLoS Comput Biol. 2010. PMID: 20300645 Free PMC article.
-
Global parameter search reveals design principles of the mammalian circadian clock.BMC Syst Biol. 2008 Feb 29;2:22. doi: 10.1186/1752-0509-2-22. BMC Syst Biol. 2008. PMID: 18312618 Free PMC article.
-
Oscillator model reduction preserving the phase response: application to the circadian clock.Biophys J. 2008 Aug;95(4):1658-73. doi: 10.1529/biophysj.107.128678. Epub 2008 May 16. Biophys J. 2008. PMID: 18487303 Free PMC article.
-
Sensitivity Measures for Oscillating Systems: Application to Mammalian Circadian Gene Network.IEEE Trans Automat Contr. 2008 Jan 1;53:177-188. doi: 10.1109/TAC.2007.911364. IEEE Trans Automat Contr. 2008. PMID: 19593456 Free PMC article.
-
Suprachiasmatic nucleus: cell autonomy and network properties.Annu Rev Physiol. 2010;72:551-77. doi: 10.1146/annurev-physiol-021909-135919. Annu Rev Physiol. 2010. PMID: 20148688 Free PMC article. Review.
References
-
- Merrow, M., T. Roenneberg, G. Macino, and L. Franchi. 2001. A fungus among us: the Neurospora crassa circadian system. Semin. Cell Dev. Biol. 12:279–285. - PubMed
-
- Lee, K., J. J. Loros, and J. C. Dunlap. 2000. Interconnected feedback loops in the Neurospora circadian system. Science. 289:107–110. - PubMed
-
- Salome, P. A., and C. R. McClung. 2004. The Arabidopsis thaliana clock. J. Biol. Rhythms. 19:425–435. - PubMed
-
- Hendricks, J. C., J. A. Williams, K. Panckeri, D. Kirk, M. Tello, J. C. Yin, and A. Sehgal. 2001. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat. Neurosci. 4:1108–1115. - PubMed
-
- Reppert, S. M., and D. R. Weaver. 2001. Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 63:647–676. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

