Invariance of single-file water mobility in gramicidin-like peptidic pores as function of pore length
- PMID: 17369423
- PMCID: PMC1868973
- DOI: 10.1529/biophysj.106.102921
Invariance of single-file water mobility in gramicidin-like peptidic pores as function of pore length
Abstract
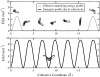
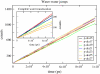
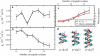
We investigated the structural and energetic determinants underlying water permeation through peptidic nanopores, motivated by recent experimental findings that indicate that water mobility in single-file water channels displays nonlinear length dependence. To address the molecular mechanism determining the observed length dependence, we studied water permeability in a series of designed gramicidin-like channels of different length using atomistic molecular dynamics simulations. We found that within the studied range of length the osmotic water permeability is independent of pore length. This result is at variance with textbook models, where the relationship is assumed to be linear. Energetic analysis shows that loss of solvation rather than specific water binding sites in the pore form the main energetic barrier for water permeation, consistent with our dynamics results. For this situation, we propose a modified expression for osmotic permeability that fully takes into account water motion collectivity and does not depend on the pore length. Different schematic barrier profiles are discussed that explain both experimental and computational interpretations, and we propose a set of experiments aimed at validation of the presented results. Implications of the results for the design of peptidic channels with desired permeation characteristics are discussed.
Figures

Similar articles
-
Determinants of water permeability through nanoscopic hydrophilic channels.Biophys J. 2009 Feb;96(3):925-38. doi: 10.1016/j.bpj.2008.09.059. Biophys J. 2009. PMID: 19186131 Free PMC article.
-
Design of peptide-membrane interactions to modulate single-file water transport through modified gramicidin channels.Biophys J. 2012 Oct 17;103(8):1698-705. doi: 10.1016/j.bpj.2012.08.059. Epub 2012 Oct 16. Biophys J. 2012. PMID: 23083713 Free PMC article.
-
Mobility of a one-dimensional confined file of water molecules as a function of file length.Phys Rev Lett. 2006 Apr 14;96(14):148101. doi: 10.1103/PhysRevLett.96.148101. Epub 2006 Apr 10. Phys Rev Lett. 2006. PMID: 16712124
-
Engineering the gramicidin channel.Annu Rev Biophys Biomol Struct. 1996;25:231-58. doi: 10.1146/annurev.bb.25.060196.001311. Annu Rev Biophys Biomol Struct. 1996. PMID: 8800470 Review.
-
Permeation in ion channels: the interplay of structure and theory.Trends Neurosci. 2004 Jun;27(6):308-14. doi: 10.1016/j.tins.2004.03.013. Trends Neurosci. 2004. PMID: 15165734 Review.
Cited by
-
Determinants of water permeability through nanoscopic hydrophilic channels.Biophys J. 2009 Feb;96(3):925-38. doi: 10.1016/j.bpj.2008.09.059. Biophys J. 2009. PMID: 19186131 Free PMC article.
-
Collective Domain Motion Facilitates Water Transport in SGLT1.Int J Mol Sci. 2023 Jun 23;24(13):10528. doi: 10.3390/ijms241310528. Int J Mol Sci. 2023. PMID: 37445706 Free PMC article.
-
Positively charged residues at the channel mouth boost single-file water flow.Faraday Discuss. 2018 Sep 28;209(0):55-65. doi: 10.1039/c8fd00050f. Faraday Discuss. 2018. PMID: 29972179 Free PMC article.
-
The energetic barrier to single-file water flow through narrow channels.Biophys Rev. 2021 Nov 23;13(6):913-923. doi: 10.1007/s12551-021-00875-w. eCollection 2021 Dec. Biophys Rev. 2021. PMID: 35035593 Free PMC article. Review.
-
Design of peptide-membrane interactions to modulate single-file water transport through modified gramicidin channels.Biophys J. 2012 Oct 17;103(8):1698-705. doi: 10.1016/j.bpj.2012.08.059. Epub 2012 Oct 16. Biophys J. 2012. PMID: 23083713 Free PMC article.
References
-
- de Groot, B. L., and H. Grubmüller. 2001. Water permeation across biological membranes: mechanism and dynamics of Aquaporin-1 and GlpF. Science. 294:2353–2357. - PubMed
-
- Roux, B., and K. Schulten. 2004. Computational studies of membrane channels. Structure. 12:1343–1351. - PubMed
-
- Roux, B. 2005. Ion conduction and selectivity in K+ channels. Annu. Rev. Biophys. Biomol. Struct. 34:153–171. - PubMed
-
- Futaki, S., Y. J. Zhang, T. Kiwada, I. Nakase, T. Yagami, S. Oiki, and Y. Sugiura. 2004. Gramicidin-based channel systems for the detection of protein-ligand interaction. Bioorg. Med. Chem. 12:1343–1350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

